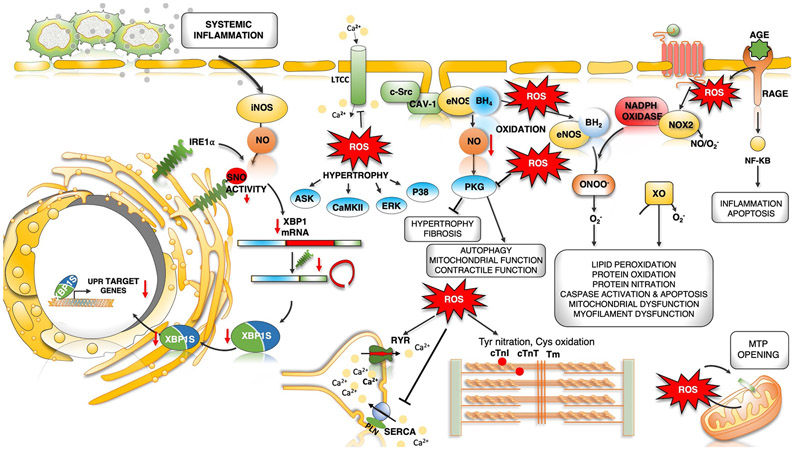
Fig. 4 ∣. Dysregulated oxidative and nitrosative stress in HFpEF pathogenesis.
Increased microvascular inflammation and pro-inflammatory cytokine levels result in increased expression of inducible nitric oxide synthase (NOS2) in cardiomyocytes. NOS2-derived nitric oxide (NO) mediates S-nitrosylation (SNO) of the unfolded protein response (UPR) regulator IRE1α, leading to a progressive decline in IRE1α-mediated generation of the spliced form of X-box-binding protein 1 (XBP1), known as XBP1s. Reduced XBP1s levels lead to decreased XBP1s-dependent expression of UPR target genes, compromised UPR and endoplasmic reticulum (ER) function, and prolonged ER stress. IRE1α activity and XBP1s levels are reduced in the hearts of patients with heart failure with preserved ejection fraction (HFpEF). Oxidative stress and increased reactive oxygen species (ROS) formation can directly modulate cardiac redox status by reacting with NO to decrease its bioavailability. Oxidative stress uncouples endothelial nitric oxide synthase (NOS3) by oxidation and depletion of its cofactor tetrahydrobiopterin (BH4) to dihydrobiopterin (BH2). NOS3-derived NO has antihypertrophic and antifibrotic effects primarily by activation of cGMP–protein kinase G (PKG) signalling. Uncoupled NOS3 generates oxidant species promoting protein tyrosine nitration, cysteine oxidation and lipid peroxidation, damaging proteins, lipids and DNA. Receptor-induced activation of NADPH oxidase 2 (NOX2) and mitochondrial redox mismatch are other major sources of ROS and stimulate mitochondrial transition pore (MTP) opening, Ca2+ overload and mitochondrial dysfunction. Advanced glycation end products (AGEs) that bind to cell surface receptors for AGEs (RAGEs) can stimulate NADPH oxidase, thereby increasing the production of ROS and aggravating inflammation by activation of the nuclear factor-κB (NF-κB) pathway. ROS can directly or indirectly activate various kinases, resulting in hypertrophy. Post-translational redox modifications of protein kinases (such as Ca2+/calmodulin-dependent protein kinase II (CAMKII), protein kinase A and PKG), sarcoplasmic reticulum (SR) proteins (ryanodine receptor 2 (RYR2) and sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2A (SERCA2A)) and myofilament proteins (cardiac troponin I (cTnI), cardiac troponin T (cTnT), tropomyosin (Tm) and cardiac myosin binding protein C can alter their activity and contribute to hypertrophy and altered excitation–contraction coupling. AngII, angiotensin II; ASK, apoptosis signal-regulating kinase; AT1R, angiotensin II receptor type 1; CAV1, caveolin 1; ERK, extracellular-signal-regulated kinase; LTCC, L-type Ca2+ channel; O2−, superoxide anion; ONOO−, peroxynitrite; PLN, cardiac phospholamban; SRC, proto-oncogene tyrosine-protein kinase SRC; XO, xanthine oxidoreductase.