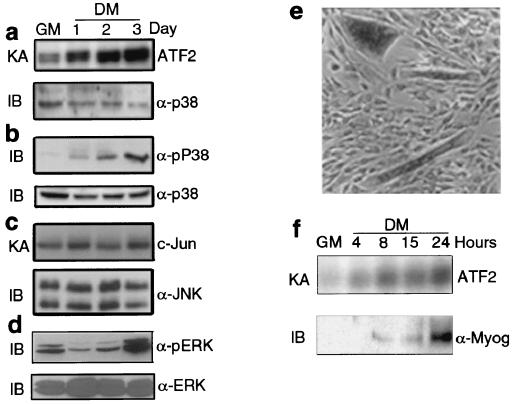
FIG. 1.
p38 activity increases upon muscle differentiation prior to induction of myogenin. (a) C2C12 myoblasts were cultured in GM until they were 90 to 95% confluent and then shifted to DM. Lysates were prepared from subconfluent cells in GM or cells kept for 1 to 3 days in DM as indicated. p38 was immunoprecipitated and its activity was measured by an immune complex kinase assay (KA) using AFT2 as a substrate. The amount of p38 was assessed by immunoblotting (IB). α-p38, anti-p38 antibody. (b) Equal amounts of whole-cell lysates were separated by SDS-PAGE in duplicate. Activation of p38 in C2C12 cell lysates was determined by immunoblotting with antibodies specific to phosphorylated and activated p38 (α-pP38) or total p38. (c) JNK activity and expression in C2C12 cell lysates were determined by an immune complex kinase assay using c-Jun as a substrate and by immunoblotting, respectively. α-JNK, anti-JNK antibody. (d) Samples (30 μg) of C2C12 extracts were analyzed by immunoblotting with anti-phospho-ERK antibody (α-pERK). The same membrane was stripped and reprobed with antibody against total ERK (α-ERK) to monitor the amounts of loaded proteins. (e) A coculture of undifferentiated C2C12 myoblasts and differentiated myotubes was prepared by mixing a population of myoblasts growing in GM and myoblasts previously cultured in DM for 24 h. This mixed population was cultured in GM for 1 day. Thereafter, cells were fixed and analyzed by immunohistochemistry with antibody specific to pP38. (f) Subconfluent C2C12 cells in GM were switched to DM and were collected at the indicated times (in hours). p38 activity was examined by an immune complex kinase assay, and expression of myogenin was determined by immunoblotting. α-Myog, anti-myogenin antibody.