Abstract
Chronic kidney disease is an important clinical condition beset with racial and ethnic disparities that are associated with social inequities. Many medical schools and health centres across the USA have raised concerns about the use of race — a socio-political construct that mediates the effect of structural racism — as a fixed, measurable biological variable in the assessment of kidney disease. We discuss the role of race and racism in medicine and outline many of the concerns that have been raised by the medical and social justice communities regarding the use of race in estimated glomerular filtration rate equations, including its relationship with structural racism and racial inequities. Although race can be used to identify populations who experience racism and subsequent differential treatment, ignoring the biological and social heterogeneity within any racial group and inferring innate individual-level attributes is methodologically flawed. Therefore, although more accurate measures for estimating kidney function are under investigation, we support the use of biomarkers for determining estimated glomerular filtration rate without adjustments for race. Clinicians have a duty to recognize and elucidate the nuances of racism and its effects on health and disease. Otherwise, we risk perpetuating historical racist concepts in medicine that exacerbate health inequities and impact marginalized patient populations.
Subject terms: Culture, End-stage renal disease, Medical ethics, Health policy
Here, the authors discuss how structural racism underlies many of the health disparities that affect individuals from minority racial groups. They also examine how the use of race coefficients in estimated glomerular filtration rate equations might contribute to health inequities in Black patients with kidney disease.
Key points
Race and ethnicity are socio-political constructs that are inextricably tied to health outcomes for individuals from racial and ethnic minority groups worldwide.
Historically, science has developed and relied on racial frames to artificially organize people into presumed homogeneous and genetically distinct racial groups, to suggest that inherent biological differences exist between the groups.
The use of race coefficients in estimated glomerular filtration rate equations reinforces flawed assumptions of race essentialism and potentially perpetuates health inequities for Black individuals with kidney disease.
Valid and race-free methods of kidney function estimation should be used to promote high-quality science, guide clinical management decisions and decrease racial bias.
Introduction
Racism can be defined as “a system of structuring opportunity and assigning value based on the social interpretation of how one looks (which is what we call ‘race’), that unfairly disadvantages some individuals and communities, unfairly advantages other individuals and communities, and saps the strength of the whole society through the waste of human resources”1. Structural racism encapsulates the historical and contemporary discriminatory laws, policies and practices that have denigrated and disinvested in Black and other oppressed communities. Given the persistent political and social underpinnings of structural racism globally, individuals from minority racial and ethnic groups predictably tend to have worse health outcomes, including all-cause mortality associated with hypertension, diabetes and chronic kidney disease (CKD), than majority populations2–7. Most recently, the coronavirus disease 2019 (COVID-19) pandemic has disproportionately affected minority racial and ethnic communities worldwide, and has magnified the global dialogue about the impact of structural racism on health8,9.
The World Health Organization affirms the global need to address the impact of racism, racial discrimination, xenophobia and related intolerance on health10. Within nephrology specifically, major efforts have been undertaken to identify and address racial and ethnic health disparities caused by structural racism across the spectrum of kidney disease11–18. However, the racism embedded within social structures and institutions is pervasive, has adversely affected Black and other marginalized groups for generations and persists to this day19–22. Notably in the USA, the public discourse includes debates about the existence of racism and denial of its existence happens at the highest level of US governance23. This denial reflects the omnipresence of American racism and the pervasiveness of racial inequality in all sectors of US society. This problem also occurs in other societies where the ubiquity of racism is contested. A 2021 report from the UK Commission on Race and Ethnic Disparities24 stated: “Put simply we no longer see a Britain where the system is deliberately rigged against ethnic minorities. The impediments and disparities do exist, they are varied, and ironically very few of them are directly to do with racism. Too often ‘racism’ is the catch-all explanation, and can be simply implicitly accepted rather than explicitly examined”.
In this Review, we use the diagnosis and management of CKD as an example to illustrate how structural racism and its manifestation as structural inequality are directly connected to adverse health outcomes, with a focus on Black individuals living in the USA. We examine how racial disparities in CKD are caused by inequities in major social determinants of health, for example, education, employment, housing, criminal justice, and access to health insurance and health care25–27, which are driven by structural racism28,29. Understanding and addressing how structural racism directly affects patients with CKD is crucial to improving the clinical care and outcomes of minority racial and ethnic groups29. In particular, we shed light on the controversy surrounding the role of race and racism in CKD outcomes, including the inappropriate use of a race-based coefficient in estimated glomerular filtration rate (eGFR) equations, and how this coefficient might contribute to racial stereotypes and inequities among Black patients with CKD.
Racial biology and racism in medicine
The concepts of race and ethnicity are deeply engrained in the social fabric of the USA, including in science and medicine12. Although these socio-political concepts and labels are widely used, race and ethnicity are distinct, nuanced concepts that should be operationalized thoughtfully30. Historically, the origin of the use of race in medicine in the USA is often linked to Carl Linnaeus, who is acknowledged as the father of modern taxonomy31. Building on work from the early 1700s by Francis Bernier, who classified humans into four major racial groups (American, European, Asian and African), Linnaeus not only reaffirmed the designation of the four ‘racial’ groups, but also assigned distinct skills, personality traits and other abilities to each racial category31. Linnaeus attributed what he deemed to be the most desirable personal traits to white Europeans (for example, muscular, gentle, sanguine, inventive and governed by laws) and the least desirable personal traits to Black Africans and other non-white individuals (for example, Black race: women without shame, crafty, indolent, negligent and governed by caprice; American Indian race: obstinate, merry, free and regulated by customs; and Asian race: melancholic, avaricious and ruled by opinions)31. This attribution of physical, cognitive and personality traits by a well-regarded physician was viewed as a ‘scientific’ justification of biological race and upheld assertions of racial inferiority32. Despite evidence of Linnaeus’ fallacious conflation of moral and biological characteristics of ‘races’, segments of the medical community constructed and perpetuated an ideology of race-based biological disease risk33–36. DNA analyses have since confirmed that all racial groups are genetically similar and that all modern human populations belong to the Homo sapiens species36,37. But, despite this knowledge and the recognition that race is a social construct, race continues to be incessantly and inaccurately treated as a simple biological variable38. However, those individuals who endorsed a rationale for race-based science often did not and do not perceive this ideology as supporting racism39–41.
No national or international consensus exists for definitions of race and ethnicity. Self-reported race and ethnicity can mean something different depending on location and cultural norms; a person’s racial identification is therefore highly subjective42,43. In the USA, race is generally socially assigned based on phenotypic appearance, whereas ethnicity is mainly defined by culture and language38,43. For instance, “white” or “Black” or “Asian” categorizations are based mainly on skin colour and facial features, whereas “Hispanic” and “Brazilian” refer to distinct cultures that encompass distinct languages or nationalities, regardless of race. Of note, the US Office of Management and Budget, which directs how the federal government classifies race and ethnicity, and the US Census still use many of the original Linnaean-based race categories of white, American Indian or Alaska Native, Asian, and Black or African American, Hawaiian or Other Pacific Islander, and Other44–47. This collection of race and ethnicity data by the US government is vital to identify race inequities but social justice advocates caution that these data must be used to dismantle structural racism rather than to perpetuate a racial caste system48,49.
Structural racism and medicine
The strong association between minority racial status and poor health outcomes is a pernicious effect of structural racism41. In the US and elsewhere, race and ethnicity are closely linked to residential segregation50,51, educational and income inequalities52,53, imbalance in community-level assets, reduced access to health-care resources54,55 and elevated exposure to environmental toxins56–58. In the USA medical profession, James McCune Smith was one of the earliest physicians to argue against theories of essential, biological racial differences in the 1840s. An abolitionist and the first African American with a medical degree, his lectures and writings postulated that health status was influenced by social conditions rather than innate racial constitution59–61. His arguments were scientifically reinforced by sociologist W.E.B. Du Bois, who in 1899 conducted the first comprehensive sociological study of Black individuals in the USA. Du Bois demonstrated that racial differences in mortality in Philadelphia could be explained by social contextual factors rather than innate racial traits62. Using participant observations, survey data and secondary data sources, Du Bois documented how discrimination, oppression, and white supremacist policies and actions (that is, based on beliefs of innate race-based superiority and inferiority) contributed to elevated levels of despair, disease and death among Black individuals63. One might consider McCune Smith and Du Bois true pioneers in building a cogent argument for understanding health inequities and showing that social factors, not inherent biological characteristics, explain racial differences in health outcomes64.
Despite the promulgation of civil rights legislation in the USA in the nineteenth and twentieth centuries (for example, the Civil Rights Bill of 1866 and the Civil Rights Act of 1964), structural racism and discrimination still exist in the twenty-first century. The challenge, however, is that contemporary structural racism, although pervasive, is much more difficult to recognize22,28,41. In medicine, additional factors such as implicit and explicit clinician biases, medical and institutional mistrust (owing to historic and ongoing mistreatment) and stereotype threat (that is, the fear of confirming negative racial stereotypes among marginalized individuals leading to poor consequences65,66) further exacerbate worsening health outcomes for Black people as well as other marginalized racial and ethnic communities2,67–70.
The detrimental impact of racism on the lives of individuals from minority racial and ethnic groups has been highlighted by scholars of critical race theory, who have used analytical tools and social action to help identify and eradicate the many forms of racism that are deeply embedded within contemporary institutions and inform the norms of society71. For example, critical race theory has highlighted racial injustices concealed within the criminal justice system and other political machines72. The ubiquity of racism is a key tenet of critical race theory and informs the approach to discerning and addressing racial inequity in biomedical institutions, which includes racial inaccuracies reified in scientific research, clinical algorithms and health system policies73. Critical race theory emphasizes the development of innovative solutions that target race-based health equity gaps — incorporating factors such as housing, education and employment — to disrupt sources of inequity and transform existing structures into more equitable institutions71. This approach can have major implications for the prevention, diagnosis and treatment of kidney disease71,74.
Biological impact of structural racism
Race-driven structural inequities and discrimination can have a biological impact and the effects of racism can therefore be examined by analysing, for example, the differences in markers of inflammation and allostatic load (that is, the activation of neural, neuroendocrine, and neuroendocrine-immune mechanisms as an adaptation to chronic stress) across different racial groups75–78. For instance, one analysis of National Health and Nutrition Examination Survey (NHANES) data found that allostatic load scores were higher in Black than in white study participants76. Of note, this racial difference in allostatic load scores was consistently pronounced between participants with higher incomes compared with those with lower incomes. The researchers postulate that persistent racial differences in allostatic load scores despite high economic status might be due to the stress of living in a racialized society76.
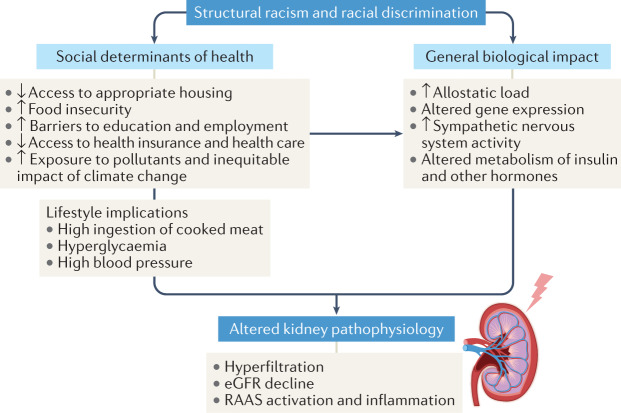
Increased allostatic load and epigenetic changes that occur in response to psychological and environmental insults, such as those endured by victims of racism, may recur through generations79–83. The Allostatic Load and Preterm Birth Conceptual Framework describes how the placental transfer of stress hormones and mediators from mother to fetus can increase the allostatic load of the child, which enhances their future risk of early uterine activation, transformation and preterm birth83. This model is based on the finding that female offspring of stressed rats have an increased incidence of preterm delivery and that the deleterious effects of stress became more pronounced with each generation84. More human studies are needed to further explore the transgenerational transmission of adverse outcomes85, although these data imply that the biological effects of racial inequality can persist. The persistence and pervasiveness of structural racism, which manifests as the institutional and individual promotion of race-based policies and practices29, compounds the biological effects of racism (Fig. 1). Rather than analyses based on inaccurate assumptions about inherent biological racial differences, more research is needed to investigate the relationship between the biological effects of racism and chronic disease disparities, including in CKD.
Fig. 1. The effects of racism on kidney pathophysiology.
Racial and ethnic differences in health conditions and/or outcomes are mainly driven by the effects of structural racism and racial discrimination. Structural racism not only creates debilitating social inequities but also induces biological alterations that contribute to disease development. However, the impact of these important factors varies among individuals of the same racial group and is not reliably captured by race alone. eGFR, estimated glomerular filtration rate; RAAS, renin–angiotensin–aldosterone system.
Racial disparities in kidney disease
Approximately 15% of the general US population has CKD, although prevalence varies between different racial and ethnic groups86. Individuals from marginalized racial and ethnic communities have a significantly higher risk of kidney failure. Specifically, the risk of developing kidney failure that requires dialysis or kidney transplantation is 2.6-fold higher in Black individuals and 1.5-fold higher in Hispanic individuals than in white individuals86. Some data also indicate that Black individuals experience more rapid disease progression than white individuals87. Factors related to structural racism that probably drive these racial inequities in the incidence of kidney failure and progression of kidney disease include, but are not limited to, experiences of neighbourhood segregation and food insecurity, inadequate control of disease risk factors and lack of access to health insurance and care26,88–90 (Fig. 1). Access to health care attenuates some (but not all) of the racial or ethnic disparities related to CKD91–93. Moreover, several studies suggest a possible inverse relationship between discrimination and eGFR94–96. One study found an association between perceived racial and gender discrimination and poor kidney function among 1,620 individuals in the Healthy Ageing in Neighbourhoods of Diversity Across the LifeSpan study95. Similarly, another study reported an inverse association between daily discrimination and cystatin C-based eGFR among a cohort of older adults (≥52 years) in the Health and Retirement Study94; a longitudinal study of over 14,000 Brazilians reported similar findings96. Stress might be an important contributing factor to this inverse relationship between discrimination and eGFR as activation of the sympathetic nervous system can trigger hyperfiltration, salt-sensitive hypertension and progressive kidney dysfunction97,98. Importantly, Black and Hispanic populations have a higher prevalence of diabetes and hypertension than other racial groups, which is a major driver of their disproportionate CKD burden. Recognizing how the effects of structural racism drive the inequitable prevalence of these comorbidities is also important86,99. Further research is needed to assess the impact of multilevel interventions, such as equitable access to nutritious foods, safe neighbourhoods, quality health care and provision of support for effective health care navigation, on racial inequities among patients at risk of or with CKD.
Of note, several gene variants have been identified as risk factors for kidney disease but they only partially explain racial disparities in kidney disease100–102. For example, ~13% of Black individuals in the USA have a high-risk APOL1 genotype, which is associated with a high prevalence and rapid progression of CKD103. High-risk APOL1 variants are typically associated with West African ancestry and do not align directly with racial delineations or broad continental ancestry, as the prevalence of APOL1 variants varies significantly around the world104–106. Thus, the presence of these genetic variants in some individuals of West African descent should not be used to make generalizations about all people who may be labelled as Black around the world. In fact, even across different ethnic groups in Nigeria, the prevalence of high-risk APOL1 variants varies from 2.1–49.1% indicating massive heterogeneity even within one of the regions with greatest prevalence107. Precision medicine in nephrology remains an area of development and substantial additional work is needed to further understand the direct link between genetic markers such as APOL1 and CKD across populations globally108.
Despite the increased prevalence of advanced CKD among Black individuals, they are less likely than white individuals to receive nephrology care prior to initiating dialysis109. Black individuals are also more likely than white individuals to start dialysis with a catheter, rather than having an arteriovenous fistula surgically placed for optimal vascular access, and are less likely to receive treatments such as home haemodialysis or peritoneal dialysis, both of which are associated with improved physical function compared with in-centre haemodialysis110–112. Black individuals are also significantly less likely than white individuals to undergo kidney transplantation113; numerous barriers contribute to this disparity, including clinician bias and the use of complicated clinical transplantation evaluation processes113,114. Additionally, a 2021 study demonstrated that the use of the race coefficient in eGFR equations can contribute to delays in attaining transplant waitlist priority among Black individuals115. Whether the use of a race coefficient has also contributed to an ‘epidemiological paradox’, whereby Black patients in the early stages of CKD are underdiagnosed yet have a disproportionately high rate of kidney failure is unclear86,116.
The use of race coefficients in eGFR equations
GFR is difficult to measure directly and can vary significantly based on the exogenous biomarker that is used, as well as the method of sample collection117,118. Clinicians therefore need estimates of kidney filtration to guide dosing of medications or diagnosing kidney disease. The most commonly used approaches to estimating GFR for these purposes are the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and the 1999 Modification of Diet in Renal Disease Study (MDRD) equations119,120. Both of these equations apply a race multiplicative coefficient of 1.16 and 1.21, respectively, for Black individuals119,120. These formulas were derived using gold standard direct measurements of kidney function based on iothalamate infusion and urinary clearance, in combination with statistical modelling to create equations with race, gender and age coefficients. The race coefficients were intended to account for the differences observed in blood creatinine levels between Black and white individuals with similar measured GFR. In the MDRD study, the authors postulated that racial differences in blood creatinine levels were likely due to Black individuals having greater muscle mass than white individuals120. The literature referenced to support this claim was not scientifically sound121–123, and subsequent studies have yet to definitively prove this assertion. The inclusion of race in eGFR equations might have been driven by the desire to achieve statistical accuracy in the quantification of kidney function but whether the methodological or ethical problems inherent in this approach were sufficiently considered remains unclear124.
Race itself is an estimated variable that cannot be accurately captured or measured. Moreover, the inclusion of race in these formulas represents an example of ecological fallacy (that is, the flawed inference of individual characteristics based on group data)125. For instance, individuals who identify or are presumed to identify as Black undoubtedly constitute a highly genetically and socially heterogeneous racial group that includes multiracial individuals126. Therefore, the introduction of a single racial modifier for all individuals who might be categorized as Black based on subjective phenotypical appearance and whom also have differential exposures to racism and its subsequent effects, is methodologically flawed127.
In addition to a review of the statistical methods used to derive eGFR equations, a closer examination of the populations included in these studies is warranted to understand their generalizability. The MDRD study enrolled 1,628 individuals; the majority were white and only 12% were Black119. Although the authors originally reported that “ethnicity was assigned by study personnel, without explicit criteria, probably by examination of skin color”128, this information was changed to “ethnicity was self-identified by the participants” in a March 2021 post-publication correction128. The CKD-EPI study featured significantly more individuals (n = 12,150) than the MDRD study and had a higher proportion of Black individuals (32% in the development cohort)120. However, race was not ascertained by self-identification for all individuals in this study and detailed methods describing how race was obtained remain unknown. Notably, 70% of Black individuals in the CKD-EPI study were participants from the African American Study of Kidney Disease and Hypertension (AASK) and had hypertensive kidney disease129, whereas individuals from other racial groups had a greater variety of kidney disease and/or normal kidney function120. Furthermore, nearly 50% of the AASK cohort had an annual income <US$15,000 and many had not completed a high school education129; similar demographic data for participants from other racial groups in eGFR equation studies remain unknown. Although appropriate representation of persons of colour in clinical research is crucial yet rarely achieved, the AASK cohort was not a representative sample of Black individuals living in the USA at around that time130,131. Some might argue that participants in eGFR studies should be representative of patients with CKD; however, eGFR formulas are used to screen individuals for disease so participant characteristics should match the general population as closely as possible. Of note, individuals living under impoverished conditions might have elevated creatinine levels owing to a variety of environmental factors, including dietary options (for example, high consumption of canned or cooked meats132,133), having occupations associated with greater muscle mass accumulation (for example, manual labour) and other repercussions of economic deprivation and oppression. However, these factors and the mechanisms by which they might affect the generation and secretion of creatinine (Fig. 2) have not yet been fully explored in eGFR studies119,120,134,135. This gap in knowledge highlights the imperative for diversification of population health studies across sociodemographic strata in addition to self-reported race. Without such data, it becomes practically impossible to ensure generalizability and extraordinarily difficult to control for the impact of structural racism in the USA136.
Fig. 2. Social determinants of creatinine metabolism.
Kidney function is commonly assessed using estimated glomerular filtration rate (eGFR) equations that are derived from blood creatinine levels. However, the generation and secretion of creatinine, and therefore, creatinine-based eGFR, can be influenced by a multitude of social factors that are highly variable among all individuals, including those of Black race.
Testing of eGFR equations in Japan and China suggested the need to use race coefficients to achieve the most accurate eGFR compared with equations developed in the US population137. For instance, one study re-evaluated the use of the MDRD equation for Chinese individuals and their analyses yielded a coefficient of 1.23 using the MDRD equation138. Another study determined a coefficient of 0.81 for Japanese individuals using the MDRD equation139. However, several differences exist in the study protocols used to determine the relationship between blood creatinine and measured GFR in the US, Japanese and Chinese populations. Methodological differences, rather than racial differences, might therefore underlie the need for these coefficients. By contrast, others found that a racial or ethnic coefficient in both MDRD and CKD-EPI equations did not improve statistical accuracy among Chinese, Malaysian and other Asian individuals140. Thus, evidence that better statistical precision can be achieved by applying a racial or ethnic coefficient for creatinine-based eGFR equations is inconsistent and highlights the flaw of using race or ethnicity as a biological variable. However, the racially adjusted eGFR formula implies that each Black individual possesses a higher GFR than a white individual with the same level of blood creatinine — independently of any factors that might induce hyperfiltration, affect muscle mass or other confounders. Importantly, to date, no studies definitively demonstrate inherent differences in measured kidney function between Black and white patients. Nonetheless, these race coefficients, which are applied to individuals, serve to reinforce the flawed idea that each racial and ethnic population is fundamentally homogeneous and that defined biological differences exist between races and ethnicities.
Several studies have tested the Black race coefficient outside of the USA — in Brazil141,142, Ghana143, Democratic Republic of the Congo and Ivory Coast144, and South Africa145 — and found that inclusion of the race coefficient did not improve the accuracy of eGFR calculations using the MDRD or CKD-EPI formulas. Furthermore, one study demonstrated that using the Black race coefficient in the CKD-EPI equation overestimated GFR among African Europeans146. Consequently, the same blood creatinine level would result in different eGFR values for a Black individual travelling to different countries, whereas the eGFR of a non-Black travelling companion would remain relatively consistent. From a pragmatic perspective, no guidelines exist to help US clinicians to manage the use of race in eGFR equations for Black patients who have immigrated from other countries where the race coefficient is not used147. The lack of reproducibility of eGFR equation accuracy across Black populations globally reinforces the notion that race is not a fixed biological entity.
Overall, the reasons underlying the variation in eGFR race coefficient data across different studies remain unclear. This variation might result from population-level differences in creatinine metabolism that reflect the effects of racism and oppression on socio-environmental factors (such as dietary practices or other lifestyle factors) or on renal pathophysiology. Alternatively, selection bias in the studies used to develop the race coefficient or other confounders might account for the variance148,149. For example, data from NHANES suggest that, although no apparent difference exists in the type of dietary protein intake by racial or ethnic group150, Black individuals had a 15% higher net urinary acid excretion than white individuals (50.9 versus 44.2 mEq/day), which implies a higher overall dietary protein intake151. Similarly, among Black Brazilians, high urinary sodium excretion was associated with increased eGFR — a finding indicating slightly higher salt consumption among Black Brazilians than that of the general Brazilian population97. These findings suggest that dietary differences or other environmental factors might induce both hyperfiltration and alter creatinine metabolism, which would increase measured GFR, inflate eGFR, and might explain the differences in eGFR observed between racial groups in the cohorts used to derive GFR-estimating equations.
Biomarkers and eGFR equations
Creatinine and cystatin C are the most widely used biomarkers in eGFR equations. However, cystatin C levels are less variable between racial or ethnic groups than creatinine levels134. Interestingly, using both cystatin C and creatinine improves the accuracy of eGFR while reducing the independent association of race with GFR118,134. Nonetheless, most available biomarkers are limited in their capability to fully capture nuances related to factors that are associated with body composition or environmental variables that can affect GFR. For instance, in the Chronic Renal Insufficiency Cohort Study, researchers found that adding body composition variables using bioelectric impedance analysis152 reduced the eGFR race coefficient for Black individuals from 16% to a negligible 3.3% when using the Chronic Renal Insufficiency Cohort eGFR equation135. Further studies are needed to better understand the association between endogenous and environmental effects on creatinine and cystatin C to optimize eGFR equation performance. In the past year, researchers have aimed to develop accurate eGFR equations based on alternative biomarkers that can be used without any racial adjustment and this work is ongoing153.
Implications of using race in eGFR equations
The rationale for developing the race coefficient as a tool to achieve more accurate eGFR formulas likely did not explicitly intend to denigrate or disadvantage Black individuals. However, the absence of valid scientific evidence to justify the use of race as a quantifiable ‘biological’ variable is unacceptable and, as noted above, race should not be used to make any biological inferences about individuals154. Despite this limitation, international guidelines recommend using CKD-EPI eGFR equations to diagnose CKD147.
A study of patients in a large US health system demonstrated that removing the race coefficient from the CKD-EPI equation resulted in 64 Black individuals being reclassified from having an eGFR >20 ml/min/1.73 m2 to an eGFR ≤20 ml/min/1.73 m2, which is the criterion established in national policy for a patient to start accumulating priority on the US kidney transplant waitlist155. Among those 64 individuals, none achieved a composite outcome of being referred, evaluated or waitlisted for kidney transplantation. Although observational data preclude definitive conclusions, these findings imply that clinicians may wait for the eGFR value to fall to at least 20 ml/min/1.73 m2 before pursuing transplant evaluation for their patients.
In general, the use of automatic adjustments for race in medicine can inadvertently cause worse outcomes for individuals from minority racial and ethnic groups73. For instance, calculators that predict risk of in-hospital mortality for patients with acute heart failure assign a lower mortality risk to Black patients (compared with non-Black patients), potentially withholding recommended medical therapy from this population73. The COVID-19 pandemic has further highlighted how current algorithms or clinical measures that do not specifically account for racial differences due to structural racism, such as life expectancy predictions that are heavily skewed by neighbourhood characteristics, might further disadvantage individuals from minority populations8,156,157. Yet, studies commonly assume racial differences are due to biology, with limited mention of contributing structural factors and without performing analyses that explicitly include these domains33,158,159.
Importantly, the lack of transparent communication with patients about the use of racially adjusted equations and algorithms violates a central principle of shared decision-making160. Additionally, the use of race as a biological construct to guide clinical care perpetuates implicit and explicit biases in clinical decision-making161 and suggests racial categorization as a risk factor for disease rather than as a risk factor for racism162.
Eliminating race coefficients from eGFR equations
The use of race when developing scientific and clinical algorithms that can be applied at the individual level must be guided by a beneficence principle, that is, the inclusion of race must be done in a manner that ensures that the process does not harm the health and well-being of patients163. Ultimately, the goal is to use the highest quality science to improve health-care delivery and account for race at a systems level to ensure health equity. Some resistance to the removal of the eGFR race coefficient is grounded in the argument that its removal might have untoward effects, such as the overdiagnosis of CKD, limited access to testing (for example, imaging procedures with radiological contrast) and treatments, inappropriate initiation of dialysis and reduction in the number of eligible Black living kidney donors164 (Table 1). For example, some data indicate that a small group of Black patients with an eGFR of 30–34 ml/min/1.73 m2 might no longer receive medications such as metformin or sodium-glucose co-transporter 2 inhibitors if the race coefficient is removed164,165. However, if the GFR of those patients has been overestimated owing to the use of a race coefficient, it is possible that the benefits of these medications might not outweigh the risks to the kidney163. Furthermore, a 2021 study estimated that if the race coefficient was removed, substantially more Black individuals with diabetes and CKD would be eligible to receive glucose-lowering medications than those who would no longer be eligible166. Regarding the eligibility to donate a kidney, the evaluation of kidney function among potential healthy individuals should not be based solely on eGFR and should take into account many factors, including the future risk of kidney disease. Furthermore, given the substantial imprecision of eGFR equations in many patients, even outside of race120,167,168, current guidelines recommend confirmatory testing, such as the use of cystatin C as a biomarker147, which should enable accurate GFR estimation without race169,170.
Table 1.
Potential implications of the removal of race from the 2009 CKD-EPI eGFR equation
| Clinical implication | eGFR threshold (ml/min/ 1.73 m2) | Estimated impacta | Additional considerations | Warranted future research |
|---|---|---|---|---|
| CKD diagnosis | <60 | ~ 1 million Black adults will be newly diagnosed with CKD | Potential for closer monitoring for CKD progression in a high-risk group | Racial disparities in early CKD diagnosis among Black adults |
| Referral to nephrologist | <30 | An additional ~68,000 Black patients will have increased specialty evaluation | More aggressive control of cardiac and kidney failure risk factors | Time to referral for Black adults before and after removing the race coefficient |
| Eligibility for kidney transplant waiting list | ≤20 | An additional ~37,000 Black patients will have timely access to transplant evaluation | Timely access to optimal treatment for a high-risk population |
Trends in proportion of transplants received among Black adults with kidney failure before and after removal of the race coefficient Time to transplant listing for Black adults before and after removal of the race coefficient |
| Health insurance coverage for medical nutrition therapy | 13–50 | An additional ~170,000 Black individuals will have access to quality nutritional education | Increased access to optimal nutritional education and/or information in a high-risk population |
Effect of using an eGFR race coefficient on eligibility to access medical nutrition services Black patient perspectives regarding new access to medical nutrition services |
| Health insurance coverage for kidney disease education | 15–29 | An additional ~47,000 Black individuals will have access to kidney failure education | Increased timely access to education about CKD and kidney failure in a high-risk population | Effect of using an eGFR race coefficient on kidney disease education referral rates and timing |
| Limited access to clinical tests (for example, imaging procedures with contrast agents) | <60 | Many of these patients might not receive testing with contrast | Potentially protective for some Black individuals given their high risk of incident kidney failure86 | Effect of using an eGFR race coefficient on eligibility for contrast imaging studies and subsequent benefits (for example, detection of illness) and risks (for example, development of contrast agent-induced acute kidney disease) |
| Possible dose reduction for ACE inhibitors, ARBs, aldosterone antagonists and direct renin inhibitorsb | <60 | Of 717,000 at-risk individuals, the number of patients who might need dose adjustments is unknown | Dose reduction should be based on blood pressure levels and the presence of hyperkalaemia | Contribution of race coefficient to changes in doses of pertinent medications and associated outcomes (for example, progression of CKD, adverse events) |
| Possible dose adjustments for other medications (for example, opioids, β-blockers, macrolides, warfarin, and low-molecular-weight heparins)c | <60 | Of 717,000 at-risk individuals, the number of patients who might need dose adjustments is unknown | Most patients are treated for clinical endpoints and consideration for dosing is usually limited to change in CKD stage | Contribution of race coefficient to changes in doses of pertinent medications and associated outcomes (for example, pain management and proportion of inadequately treated infections) |
| Impact on patient-centred outcomes and health equity | NA | NA |
At the patient level: potential adverse impact on life insurance from a new diagnosis At the provider level: more aggressive care due to lower reported eGFR At the society level: reduced implicit and explicit racial biases in the clinical encounter; increased awareness of the legacy of racism in medicine; reconciliation towards rebuilding trust with patients and communities who have been marginalized |
Patient awareness and perspectives regarding the use of race in eGFR equations Provider understanding of the impact of the race coefficient on health equity and other clinical outcomes Implicit bias among clinicians and impact on kidney care quality metrics183 for the treatment of Black adults with and at risk of kidney disease |
ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NA, not applicable. The table is based on data from a cohort of 9,522 non-pregnant Black adults derived from the National Health and Nutrition Examination Survey 2001–2018; the proportions and confidence intervals are adjusted to be representative of the US population using the National Health and Nutrition Examination Survey weights and design, and US census data. aBased on data from Diao et al.164. bRecommendations from Kidney Disease: Improving Global Outcomes for eGFR <45 ml/min/1.73 m2: dose reduction for ACE inhibitors, ARBs, aldosterone antagonists and direct renin inhibitors. cRecommendations from Kidney Disease: Improving Global Outcomes for eGFR <30 ml/min/1.73 m2: contraindication for metformin, sodiumglucose co-transporter 2 inhibitors, cisplatin and NSAIDs; dose reduction for β-blockers, macrolides, warfarin and low-molecular-weight heparins.
By contrast, the potential advantages of removing the race coefficient include earlier awareness of CKD and more timely referral to general and transplant nephrology care for prevention or treatment163. In patients with advanced CKD, the impact of the race coefficient on eGFR levels (for example, eGFR of 12 ml/min/1.73 m2 versus 14 ml/min/1.73 m2) is probably less relevant to patient management than that of clinical factors such as medication use, level of blood pressure control, volume status or dietary protein intake171. By contrast, at higher levels of eGFR (60–70 ml/min/1.73 m2), removing the race coefficient might impact clinical decisions substantially and would affect a much higher percentage of the CKD population172 (as many as 1 million or more Black individuals with early-stage CKD164). The concerns of eliminating the eGFR race coefficient focus mainly on patients with advanced CKD, but, considering that some Black individuals are at an increased risk of CKD progression, formulas that increase eGFR to nearly 16–21% at mild stages of CKD might unintentionally delay appropriate early disease detection, risk factor management and aggressive intervention115,126,155,164,167,173.
The concerns raised about the removal of the eGFR equation race coefficient can therefore be addressed by ensuring that clinical decision-making takes into account each patient’s body habitus, comorbidities and lifestyle considerations168,174. Where medications for patients with eGFR near the threshold of 30 ml/min/1.73 m2 are concerned, the best approach might involve seeking guidance from pharmacy or nephrology, or reflex testing of cystatin C, instead of relying on a single GFR estimation172. Future guidelines should also re-emphasize the need for confirmatory testing of kidney function at specific eGFR thresholds, which should further offset any negative effects of removing race from eGFR equations through the better identification and management of kidney disease163. Hypothetically, rejecting the fallacy of Black individuals possessing greater muscle mass than individuals from every other racial group119 and removing race from eGFR or other bedside clinical tools might help in the process of reducing implicit and explicit racial biases during the clinical encounter. This change would also prompt an acknowledgement of the legacy of racism in medicine and support the process of rebuilding trust with patients, communities and clinicians who have been persistently marginalized.
Conclusions
The existence of differences in health outcomes according to racial and ethnic groupings should prompt clinicians to ask more questions. Rather than sharing a biological identity that is distinct from other racial groups, individuals from minority racial groups share a high risk of being harmed and disadvantaged by racism. Therefore, racial differences in health outcomes might reflect health inequities — including biological alterations that affect gene expression, hormone secretion and sympathetic nervous system activation — caused by political, social and environmental systems created and sustained by structural racism38. These considerations provide a framework for understanding the interplay of race and structural racism, and its impact on CKD. Importantly, although racism can have a biological impact, race remains an inaccurate surrogate for the specific sequelae of racism that might influence disease development, progression and treatment response175. Therefore, more research is needed to identify the mechanisms underlying the biological consequences of racism and its interplay with social determinants of health, so that clinical algorithms can incorporate relevant mechanistic variables that are specific to individuals, rather than use broad racial group generalizations that do not reflect their individual biology.
Although progress has been made in reducing some racial inequities in nephrology care176, the notion that the use of a Black race coefficient in eGFR equations contributes to health inequity for Black patients has not been universally accepted and has caused controversy118,126,163,164,167,177–180. Given this national discourse, the American Society of Nephrology and National Kidney Foundation convened a joint Race and eGFR Task Force to focus on the use of race to estimate GFR in the summer of 2020 (ref.180). In September 2021, the Task Force recommended immediate implementation of the 2021 CKD-EPI equation, which was refitted without race181,182.They also recommended that national efforts focus on increasing access to cystatin C and promoting research aimed at eliminating racial disparities in kidney disease181. Consistent with these recommendations, we affirm that the inconsistent performance of eGFR equations across racial and ethnic groups, substantial data demonstrating the harms of using the race coefficient and, most importantly, the methodological flaws of using race as a biological construct, warrant race-free CKD-EPI eGFR equations with creatinine, cystatin c or cystatin–creatinine. Cystatin C or combined cystatin–creatinine-based eGFR equations should be used in the context of cystatin C availability, standardization, clinician familiarity and costs. Where cystatin C is currently available, reflex testing for impactful decision-making (for example, dosing of drugs) should be considered174. Importantly, the field should focus on identifying better disease biomarkers and investigating the potential benefits of adding body composition markers to existing equations153.
The USA has reached a crossroads that requires confronting race and racism as a mandatory step to advancing health equity — it will either become the nation it claims to be, where all Americans are created equal and, by extension, receive the health care essential to enjoying liberty and pursuing happiness, or it will continue to perpetuate a racial caste system where oppressed racial and ethnic groups experience the most severe health inequities. In biomedicine, a persistent avoidance of the social and moral costs of longstanding race-based inequities prevails. The late Civil Rights icon, John Lewis, once said “When you see something that is not right, not fair, not just, you have to speak up. You have to say something — you have to do something.” Now is the time to stop the inappropriate use of race in clinical algorithms in nephrology and beyond.
Acknowledgements
N.D.E. is supported by NIH research grant K23DK114526. R.J.T. is supported in part by NIH research grants U54MD000214, K02AG059140 and R01AG054363. M.A.B. is supported in part by NIH Grants K02AG059140, R25HL126145 and P30AG059298. K.C.N. is supported in part by NIH research grants UL1TR001881 and P30AG021684.
Glossary
- Chronic Renal Insufficiency Cohort eGFR equation
An eGFR equation developed among the Chronic Renal Insufficiency Cohort that is largely restricted to research investigations.
Author contributions
N.D.E., L.E.B. and K.C.N. researched data for the article, made substantial contributions to discussions of the content and wrote the article. All authors reviewed or edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Nephrology thanks P. Delanaye, M. Hoenig and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones CP. Confronting institutionalized racism. Phylon. 2003;50:7–22. [Google Scholar]
- 2.Williams DR, Mohammed SA. Racism and health I: pathways and scientific evidence. Am. Behav. Sci. 2013;57:1152–1173. doi: 10.1177/0002764213487340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DR, Mohammed SA. Racism and health II: a needed research agenda for effective interventions. Am. Behav. Sci. 2013;57:1200–1226. doi: 10.1177/0002764213487341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gee GC, Ford CL. Structural racism and health inequities: old issues, new directions. Du. Bois Rev. 2011;8:115–132. doi: 10.1017/S1742058X11000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkinson E, Brettle A, Waqar M, Randhawa G. Inequalities and outcomes: end stage kidney disease in ethnic minorities. BMC Nephrol. 2019;20:234. doi: 10.1186/s12882-019-1410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Garcia G, Jha V. CKD in disadvantaged populations. Kidney Int. 2015;87:251–253. doi: 10.1038/ki.2014.369. [DOI] [PubMed] [Google Scholar]
- 7.Moosa MR, Norris KC. Sustainable social development: tackling poverty to achieve kidney health equity. Nat. Rev. Nephrol. 2021;17:3–4. doi: 10.1038/s41581-020-00342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogedegbe G, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw. Open. 2020;3:e2026881. doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan L, Ogunwole SM, Cooper LA. Historical insights on coronavirus disease 2019 (COVID-19), the 1918 influenza pandemic, and racial disparities: illuminating a path forward. Ann. Intern. Med. 2020;173:474–481. doi: 10.7326/M20-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO’s Contribution to the World Conference against Racism, Racial Discrimination, Xenophobia and Related Intolerance: Health and freedom from discrimination (2001).
- 11.Crews DC, Wesson DE. Persistent bias: a threat to diversity among health care leaders. Clin. J. Am. Soc. Nephrol. 2018;13:1757–1759. doi: 10.2215/CJN.07290618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norris KC, et al. Hemodialysis disparities in African Americans: the deeply integrated concept of race in the social fabric of our society. Semin. Dialysis. 2017;30:213–223. doi: 10.1111/sdi.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes, and care of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2014;23:298–305. doi: 10.1097/01.mnh.0000444822.25991.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews DC, Bello AK, Saadi G. Burden, access, and disparities in kidney disease. Kidney Int. 2019;95:242–248. doi: 10.1016/j.kint.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Semin. Nephrol. 2013;33:409–415. doi: 10.1016/j.semnephrol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Kidney Foundation. Statement on racial violence and disparities in America. kidney.orghttps://www.kidney.org/news/statement-racial-violence-and-disparities-america (2020).
- 17.American Society of Nephrology. ASN statement against racism. asn-online.orghttps://www.asn-online.org/news/item.aspx?ID=173 (2020).
- 18.Mohottige, D., Diamantidis, C. J., Norris, K. C. & Boulware, L. E. Time to repair the effects of racism on kidney health inequity. Am. J. Kidney Dis. 10.1053/j.ajkd.2021.01.010 (2021). [DOI] [PubMed]
- 19.Csete J, et al. Public health and international drug policy. Lancet. 2016;387:1427–1480. doi: 10.1016/S0140-6736(16)00619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banys P. Mitigation of marijuana-related legal harms to youth in California. J. Psychoact. Drugs. 2016;48:11–20. doi: 10.1080/02791072.2015.1126770. [DOI] [PubMed] [Google Scholar]
- 21.Chandra A, Frakes M, Malani A. Challenges to reducing discrimination and health inequity through existing civil rights laws. Health Aff. 2017;36:1041–1047. doi: 10.1377/hlthaff.2016.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey ZD, Feldman JM, Bassett MT. How structural racism works — racist policies as a root cause of U.S. Racial health inequities. N. Engl. J. Med. 2020;384:768–773. doi: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White House. Executive Order on advancing racial equity and support for underserved communities through the federal government. whitehouse.govhttps://www.whitehouse.gov/briefing-room/presidential-actions/2021/01/20/executive-order-advancing-racial-equity-and-support-for-underserved-communities-through-the-federal-government/ (2021).
- 24.The report of the Commission on Race and Ethnic Disparities. UK Government publicationshttps://www.gov.uk/government/publications/the-report-of-the-commission-on-race-and-ethnic-disparities (2021).
- 25.Hall YN. Social determinants of health: addressing unmet needs in nephrology. Am. J. Kidney Dis. 2018;72:582–591. doi: 10.1053/j.ajkd.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Norton JM, et al. Social determinants of racial disparities in CKD. J. Am. Soc. Nephrol. 2016;27:2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv. Chronic Kidney Dis. 2015;22:6–15. doi: 10.1053/j.ackd.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey ZD, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 29.Bruce MA, et al. Social environmental stressors, psychological factors, and kidney disease. J. Investig. Med. 2009;57:583–589. doi: 10.231/JIM.0b013e31819dbb91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun L, Wentz A, Baker R, Richardson E, Tsai J. Racialized algorithms for kidney function: erasing social experience. Soc. Sci. Med. 2021;268:113548. doi: 10.1016/j.socscimed.2020.113548. [DOI] [PubMed] [Google Scholar]
- 31.Linnaeus, C. Systema naturae 10th edn (1758).
- 32.Harawa NT, Ford CL. The foundation of modern racial categories and implications for research on black/white disparities in health. Ethn. Dis. 2009;19:209–217. [PubMed] [Google Scholar]
- 33.Bunyavanich S, Grant C, Vicencio A. Racial/ethnic variation in nasal gene expression of transmembrane serine protease 2 (TMPRSS2) JAMA. 2020;324:1–2. doi: 10.1001/jama.2020.17386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 35.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Panza JA. Racial differences in nitric oxide-mediated vasodilator response to mental stress in the forearm circulation. Hypertension. 1998;31:1235–1239. doi: 10.1161/01.hyp.31.6.1235. [DOI] [PubMed] [Google Scholar]
- 36.No Authors Listed. ASHG denounces attempts to link genetics and racial supremacy. Am. J. Hum. Genet. 2018;103:636. doi: 10.1016/j.ajhg.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall E. DNA studies challenge the meaning of race. Science. 1998;282:654–655. doi: 10.1126/science.282.5389.654. [DOI] [PubMed] [Google Scholar]
- 38.Ford CL, Harawa NT. A new conceptualization of ethnicity for social epidemiologic and health equity research. Soc. Sci. Med. 2010;71:251–258. doi: 10.1016/j.socscimed.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl Acad. Sci. USA. 2016;113:4296–4301. doi: 10.1073/pnas.1516047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulman KA, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N. Engl. J. Med. 1999;340:618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 41.No Authors Listed. US Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (eds Smedley, B. D. et al.) (US National Academies Press, 2003). [PubMed]
- 42.Egede LE. Race, ethnicity, culture, and disparities in health care. J. Gen. Intern. Med. 2006;21:667–669. doi: 10.1111/j.1525-1497.2006.0512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oppenheimer GM. Paradigm lost: race, ethnicity, and the search for a new population taxonomy. Am. J. Public Health. 2001;91:1049–1055. doi: 10.2105/ajph.91.7.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Office of Management and Budget. Standards for the classification of federal data on race and ethnicity. Fed. Regist. 59, 29831–29835 (1994).
- 45.Office of Management and Budget. Revisions to the standards for the classification of federal data on race and ethnicity. Fed. Regist. 1997;62:58781–58790. [Google Scholar]
- 46.Wallman KK, Evinger S, Schechter S. Measuring our nation’s diversity: developing a common language for data on race/ethnicity. Am. J. Public Health. 2000;90:1704–1708. doi: 10.2105/ajph.90.11.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Census Bureau. data.census.govhttps://data.census.gov/cedsci/profile?q=United%20States&g=0100000US (2021).
- 48.Yudell M, Roberts D, DeSalle R, Tishkoff S. Science and society. Taking race out of human genetics. Science. 2016;351:564–565. doi: 10.1126/science.aac4951. [DOI] [PubMed] [Google Scholar]
- 49.Isabel Wilkerson. America’s Enduring Caste System. Nytimes.comhttps://www.nytimes.com/2020/07/01/magazine/isabel-wilkerson-caste.html (2020).
- 50.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen JT, et al. Mapping and measuring social disparities in premature mortality: the impact of census tract poverty within and across Boston neighborhoods, 1999–2001. J. Urban. Health. 2006;83:1063–1084. doi: 10.1007/s11524-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plantinga LC, Johansen KL, Schillinger D, Powe NR. Lower socioeconomic status and disability among US adults with chronic kidney disease, 1999–2008. Prev. Chronic Dis. 2012;9:E12. [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmerman FJ, Anderson NW. Trends in health equity in the United States by race/ethnicity, sex, and income, 1993–2017. JAMA Netw. Open. 2019;2:e196386. doi: 10.1001/jamanetworkopen.2019.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powe NR. Let’s get serious about racial and ethnic disparities. J. Am. Soc. Nephrol. 2008;19:1271–1275. doi: 10.1681/ASN.2008040358. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez RA, et al. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann. Intern. Med. 2007;146:493–501. doi: 10.7326/0003-4819-146-7-200704030-00005. [DOI] [PubMed] [Google Scholar]
- 56.Gostin LO. Politics and public health: the flint drinking water crisis. Hastings Cent. Rep. 2016;46:5–6. doi: 10.1002/hast.598. [DOI] [PubMed] [Google Scholar]
- 57.White BM, Bonilha HS, Ellis C., Jr Racial/ethnic differences in childhood blood lead levels among children <72 months of age in the United States: a systematic review of the literature. J. Racial Ethn. Health Disparities. 2016;3:145–153. doi: 10.1007/s40615-015-0124-9. [DOI] [PubMed] [Google Scholar]
- 58.Tessum CW, et al. Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc. Natl Acad. Sci. USA. 2019;116:6001–6006. doi: 10.1073/pnas.1818859116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krieger N. Shades of difference: theoretical underpinnings of the medical controversy on black/white differences in the United States, 1830–1870. Int. J. Health Serv. 1987;17:259–278. doi: 10.2190/DBY6-VDQ8-HME8-ME3R. [DOI] [PubMed] [Google Scholar]
- 60.Link EP. The civil rights activities of three great negro physicians (1840–1940) J. Negro Hist. 1967;52:169–184. [PubMed] [Google Scholar]
- 61.Smith JM. On the fourteenth query of Thomas Jefferson’s notes on Virginia. Anglo-Afr. Mag. 1859;1:225–238. [Google Scholar]
- 62.Du Bois, W. E. B. The Philadelphia Negro: A Social Study (University of Pennsylvania, 1899).
- 63.DuBois WE. The health and physique of the Negro American. 1906. Am. J. Public Health. 2003;93:272–276. doi: 10.2105/ajph.93.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuberi, T. Thicker Than Blood: How Racial Statistics Lie (University of Minnesota Press, 2001).
- 65.Steele CM, Aronson J. Stereotype threat and the intellectual test performance of African Americans. J. Pers. Soc. Psychol. 1995;69:797–811. doi: 10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- 66.Aronson J, Burgess D, Phelan SM, Juarez L. Unhealthy interactions: the role of stereotype threat in health disparities. Am. J. Public Health. 2013;103:50–56. doi: 10.2105/AJPH.2012.300828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams MV, et al. Inadequate functional health literacy among patients at two public hospitals. JAMA. 1995;274:1677–1682. [PubMed] [Google Scholar]
- 68.Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep. 2003;118:358–365. doi: 10.1016/S0033-3549(04)50262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdou CM, Fingerhut AW, Jackson JS, Wheaton F. Healthcare stereotype threat in older adults in the health and retirement study. Am. J. Prev. Med. 2016;50:191–198. doi: 10.1016/j.amepre.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenwood BN, Hardeman RR, Huang L, Sojourner A. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc. Natl Acad. Sci. USA. 2020;117:21194–21200. doi: 10.1073/pnas.1913405117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ford CL, Airhihenbuwa CO. Commentary: just what is critical race theory and what’s it doing in a progressive field like public health? Ethn. Dis. 2018;28:223–230. doi: 10.18865/ed.28.S1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delgado RSJ. Critical race theory and criminal justice. Hum. Soc. 2007;31:133–145. [Google Scholar]
- 73.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight — reconsidering the use of race correction in clinical algorithms. N. Engl. J. Med. 2020;383:874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 74.Ford, C. L. & Airhihenbuwa, C. O. Critical race theory, race equity, and public health: toward antiracism praxis. Am. J. Public Health100 (Suppl 1), S30–S35 (2010). [DOI] [PMC free article] [PubMed]
- 75.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 76.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ong AD, Williams DR. Lifetime discrimination, global sleep quality, and inflammation burden in a multiethnic sample of middle-aged adults. Cultur. Divers. Ethn. Minor. Psychol. 2019;25:82–90. doi: 10.1037/cdp0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown KM, et al. Social regulation of inflammation related gene expression in the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2020;117:104654. doi: 10.1016/j.psyneuen.2020.104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Portha B, Grandjean V, Movassat J. Mother or father: who is in the front line? Mechanisms underlying the non-genomic transmission of obesity/diabetes via the maternal or the paternal line. Nutrients. 2019;11:233. doi: 10.3390/nu11020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sales VM, Ferguson-Smith AC, Patti ME. Epigenetic mechanisms of transmission of metabolic disease across generations. Cell Metab. 2017;25:559–571. doi: 10.1016/j.cmet.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Steenwyk G, Roszkowski M, Manuella F, Franklin TB, Mansuy IM. Transgenerational inheritance of behavioral and metabolic effects of paternal exposure to traumatic stress in early postnatal life: evidence in the 4th generation. Environ. Epigenet. 2018;4:dvy023. doi: 10.1093/eep/dvy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang W, et al. Prenatal famine exposure and estimated glomerular filtration rate across consecutive generations: association and epigenetic mediation in a population-based cohort study in Suihua China. Aging. 2020;12:12206–12221. doi: 10.18632/aging.103397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olson DM, et al. Allostatic load and preterm birth. Int. J. Mol. Sci. 2015;16:29856–29874. doi: 10.3390/ijms161226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao Y, et al. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med. 2014;12:121. doi: 10.1186/s12916-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christiaens I, Hegadoren K, Olson DM. Adverse childhood experiences are associated with spontaneous preterm birth: a case-control study. BMC Med. 2015;13:124. doi: 10.1186/s12916-015-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saran, R. et al. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 75, (1 Suppl 1), A6–A7 (2020). [DOI] [PubMed]
- 87.Hounkpatin HO, et al. Ethnic minority disparities in progression and mortality of pre-dialysis chronic kidney disease: a systematic scoping review. BMC Nephrol. 2020;21:217. doi: 10.1186/s12882-020-01852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johns TS, et al. Neighborhood socioeconomic status, race, and mortality in young adult dialysis patients. J. Am. Soc. Nephrol. 2014;25:2649–2657. doi: 10.1681/ASN.2013111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Volkova N, et al. Neighborhood poverty and racial differences in ESRD incidence. J. Am. Soc. Nephrol. 2008;19:356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurella-Tamura M, Goldstein BA, Hall YN, Mitani AA, Winkelmayer WC. State medicaid coverage, ESRD incidence, and access to care. J. Am. Soc. Nephrol. 2014;25:1321–1329. doi: 10.1681/ASN.2013060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Evans K, et al. Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol. Dial. Transpl. 2011;26:899–908. doi: 10.1093/ndt/gfq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krop JS, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch. Intern. Med. 1999;159:1777–1783. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 93.Perneger TV, Whelton PK, Klag MJ. Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch. Intern. Med. 1995;155:1201–1208. [PubMed] [Google Scholar]
- 94.Cobb RJ, Thorpe RJ, Norris KC. Everyday discrimination and kidney function among older adults: evidence from the Health and Retirement Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020;75:517–521. doi: 10.1093/gerona/glz294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beydoun MA, et al. Perceived discrimination and longitudinal change in kidney function among urban adults. Psychosom. Med. 2017;79:824–834. doi: 10.1097/PSY.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Camelo LV, et al. Racial disparities in renal function: the role of racial discrimination. The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) J. Epidemiol. Commun. Health. 2018;72:1027–1032. doi: 10.1136/jech-2018-210665. [DOI] [PubMed] [Google Scholar]
- 97.Santos EMD, et al. Association between estimated glomerular filtration rate and sodium excretion in urine of African descendants in Brazil: a population-based study. J. Bras. Nefrol. 2018;40:248–255. doi: 10.1590/2175-8239-JBN-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stewart DL, Harshfield GA, Zhu H, Hanevold CD. Stress and salt sensitivity in primary hypertension. Curr. Hypert. Rep. 2015;17:2. doi: 10.1007/s11906-014-0513-1. [DOI] [PubMed] [Google Scholar]
- 99.Centers for Disease Control and Prevention. Diabetes Report Card 2019 (Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2020).
- 100.Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olaniran KO, et al. Kidney function decline among black patients with sickle cell trait and sickle cell disease: an observational cohort study. J. Am. Soc. Nephrol. 2020;31:393–404. doi: 10.1681/ASN.2019050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Datta S, et al. Kidney disease-associated APOL1 variants have dose-dependent, dominant toxic gain-of-function. J. Am. Soc. Nephrol. 2020;31:2083–2096. doi: 10.1681/ASN.2020010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gutiérrez OM, et al. APOL1 nephropathy risk variants and incident cardiovascular disease events in community-dwelling black adults. Circ. Genom. Precis. Med. 2018;11:e002098. doi: 10.1161/CIRCGEN.117.002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang H, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am. J. Hum. Genet. 2005;76:268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Micheletti SJ, et al. Genetic consequences of the transatlantic slave trade in the Americas. Am. J. Hum. Genet. 2020;107:265–277. doi: 10.1016/j.ajhg.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nadkarni GN, et al. Worldwide frequencies of APOL1 renal risk variants. N. Engl. J. Med. 2018;379:2571–2572. doi: 10.1056/NEJMc1800748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wudil UJ, et al. Apolipoprotein-1 risk variants and associated kidney phenotypes in an adult HIV cohort in Nigeria. Kidney Int. 2021;100:146–154. doi: 10.1016/j.kint.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cerdeña JP, Tsai J, Grubbs V. APOL1, black race, and kidney disease: turning attention to structural racism. Am. J. Kidney Dis. 2021;77:857–860. doi: 10.1053/j.ajkd.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 109.Yan G, et al. The associations between race and geographic area and quality-of-care indicators in patients approaching ESRD. Clin. J. Am. Soc. Nephrol. 2013;8:610–618. doi: 10.2215/CJN.07780812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zarkowsky DS, et al. Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg. 2015;150:529–536. doi: 10.1001/jamasurg.2015.0287. [DOI] [PubMed] [Google Scholar]
- 111.Mehrotra R, et al. Racial and ethnic disparities in use of and outcomes with home dialysis in the United States. J. Am. Soc. Nephrol. 2016;27:2123–2134. doi: 10.1681/ASN.2015050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eneanya ND, et al. Longitudinal patterns of health-related quality of life and dialysis modality: a national cohort study. BMC Nephrol. 2019;20:7. doi: 10.1186/s12882-018-1198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Purnell TS, et al. Association of race and ethnicity with live donor kidney transplantation in the United States from 1995 to 2014. JAMA. 2018;319:49–61. doi: 10.1001/jama.2017.19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reese PP, et al. Racial disparities in preemptive waitlisting and deceased donor kidney transplantation: ethics and solutions. Am. J. Transplant. 2021;21:958–967. doi: 10.1111/ajt.16392. [DOI] [PubMed] [Google Scholar]
- 115.Zelnick LR, Leca N, Young B, Bansal N. Association of the estimated glomerular filtration rate with vs without a coefficient for race with time to eligibility for kidney transplant. JAMA Netw. Open. 2021;4:e2034004. doi: 10.1001/jamanetworkopen.2020.34004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delanaye P, Mariat C, Maillard N, Krzesinski JM, Cavalier E. Are the creatinine-based equations accurate to estimate glomerular filtration rate in African American populations? Clin. J. Am. Soc. Nephrol. 2011;6:906–912. doi: 10.2215/CJN.10931210. [DOI] [PubMed] [Google Scholar]
- 117.Porrini E, et al. Estimated GFR: time for a critical appraisal. Nat. Rev. Nephrol. 2019;15:177–190. doi: 10.1038/s41581-018-0080-9. [DOI] [PubMed] [Google Scholar]
- 118.Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin. J. Am. Soc. Nephrol. 2020;15:1203–1212. doi: 10.2215/CJN.12791019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levey AS, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 120.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cohn SH, et al. Body elemental composition: comparison between black and white adults. Am. J. Physiol. 1977;232:E419–E422. doi: 10.1152/ajpendo.1977.232.4.E419. [DOI] [PubMed] [Google Scholar]
- 122.Harsha DW, Frerichs RR, Berenson GS. Densitometry and anthropometry of black and white children. Hum. Biol. 1978;50:261–280. [PubMed] [Google Scholar]
- 123.Worrall JG, Phongsathorn V, Hooper RJ, Paice EW. Racial variation in serum creatine kinase unrelated to lean body mass. Br. J. Rheumatol. 1990;29:371–373. doi: 10.1093/rheumatology/29.5.371. [DOI] [PubMed] [Google Scholar]
- 124.Levey AS, Tighiouart H, Titan SM, Inker LA. Estimation of glomerular filtration rate with vs without including patient race. JAMA Intern. Med. 2020;180:793–795. doi: 10.1001/jamainternmed.2020.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwartz S. The fallacy of the ecological fallacy: the potential misuse of a concept and the consequences. Am. J. Public Health. 1994;84:819–824. doi: 10.2105/ajph.84.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322:113–114. doi: 10.1001/jama.2019.5774. [DOI] [PubMed] [Google Scholar]
- 127.Subramanian SV, Jones K, Kaddour A, Krieger N. Revisiting Robinson: the perils of individualistic and ecologic fallacy. Int. J. Epidemiol. 2009;38:342–360. doi: 10.1093/ije/dyn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levey AS, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 129.Sika M, et al. Baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Clinical Trial and Cohort Study. Am. J. Kidney Dis. 2007;50:78–89. doi: 10.1053/j.ajkd.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 130.Kochhar, R. and Cilluffo, A. Appendix A: Income distributions of whites, blacks, Hispanics and Asians in the U.S., 1970 and 2016. pewresearch.orghttps://www.pewsocialtrends.org/2018/07/12/appendix-a-income-distributions-of-whites-blacks-hispanics-and-asians-in-the-u-s-1970-and-2016/ (2018).
- 131.DeNavas-Walt, C., Proctor B. D. & Smith J. C. Income Poverty and Health Insurance Coverage in the United States: 2011 (U.S Census Bureau, 2011).
- 132.Diskin CJ. Creatinine and glomerular filtration rate: evolution of an accommodation. Ann. Clin. Biochem. 2007;44:16–19. doi: 10.1258/000456307779595940. [DOI] [PubMed] [Google Scholar]
- 133.Nair S, et al. Effect of a cooked meat meal on serum creatinine and estimated glomerular filtration rate in diabetes-related kidney disease. Diabetes Care. 2014;37:483–487. doi: 10.2337/dc13-1770. [DOI] [PubMed] [Google Scholar]
- 134.Inker LA, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anderson AH, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eneanya ND, Kostelanetz S, Mendu MM. Race-free biomarkers to quantify kidney function: health equity lessons learned from population-based research. Am. J. Kidney Dis. 2021;77:667–669. doi: 10.1053/j.ajkd.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Rule AD, Teo BW. GFR estimation in Japan and China: what accounts for the difference? Am. J. Kidney Dis. 2009;53:932–935. doi: 10.1053/j.ajkd.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma YC, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 139.Matsuo S, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 140.Teo BW, et al. GFR estimating equations in a multiethnic Asian population. Am. J. Kidney Dis. 2011;58:56–63. doi: 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- 141.Barcellos RC, Matos JP, Kang HC, Rosa ML, Lugon JR. Comparison of serum creatinine levels in different color/race categories in a Brazilian population. Cad. Saude Publica. 2015;31:1565–1569. doi: 10.1590/0102-311X00150814. [DOI] [PubMed] [Google Scholar]
- 142.Zanocco JA, et al. Race adjustment for estimating glomerular filtration rate is not always necessary. Nephron Extra. 2012;2:293–302. doi: 10.1159/000343899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Eastwood JB, et al. Assessment of GFR by four methods in adults in Ashanti, Ghana: the need for an eGFR equation for lean African populations. Nephrol. Dial. Transplant. 2010;25:2178–2187. doi: 10.1093/ndt/gfp765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bukabau JB, et al. Performance of creatinine- or cystatin C-based equations to estimate glomerular filtration rate in sub-Saharan African populations. Kidney Int. 2019;95:1181–1189. doi: 10.1016/j.kint.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 145.van Deventer HE, George JA, Paiker JE, Becker PJ, Katz IJ. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin. Chem. 2008;54:1197–1202. doi: 10.1373/clinchem.2007.099085. [DOI] [PubMed] [Google Scholar]
- 146.Flamant M, et al. Performance of GFR estimating equations in African Europeans: basis for a lower race-ethnicity factor than in African Americans. Am. J. Kidney Dis. 2013;62:182–184. doi: 10.1053/j.ajkd.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 147.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 148.Miller W. Perspective on new equations for estimating glomerular filtration rate. Clin. Chem. 2021;67:820–822. doi: 10.1093/clinchem/hvab029. [DOI] [PubMed] [Google Scholar]
- 149.Schmidt IM, Waikar SS. Separate and unequal: race-based algorithms and implications for nephrology. J. Am. Soc. Nephrol. 2021;32:529–533. doi: 10.1681/ASN.2020081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beasley JM, Firestone MJ, Popp CJ, Russo R, Yi SS. Age and racial/ethnic differences in dietary sources of protein, NHANES, 2011–2016. Front. Nutr. 2020;7:76. doi: 10.3389/fnut.2020.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Crews DC, et al. Race/ethnicity, dietary acid load, and risk of end-stage renal disease among US adults with chronic kidney disease. Am. J. Nephrol. 2018;47:174–181. doi: 10.1159/000487715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am. J. Clin. Nutr. 1988;48:16–23. doi: 10.1093/ajcn/48.1.16. [DOI] [PubMed] [Google Scholar]
- 153.Inker LACS, Tighiouart H, et al. A new panel estimated GFR, including B2-microglobulin and B-trace protein and not including race, developed in a diverse population. Am. J. Kidney Dis. 2020;77:673–683.e1. doi: 10.1053/j.ajkd.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robinson WS. Ecological correlations and the behavior of individuals. Int. J. Epidemiol. 2009;38:337–341. doi: 10.1093/ije/dyn357. [DOI] [PubMed] [Google Scholar]
- 155.Ahmed S, et al. Examining the potential impact of race multiplier utilization in estimated glomerular filtration rate calculation on African-American care outcomes. J. Gen. Intern. Med. 2021;36:464–471. doi: 10.1007/s11606-020-06280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cleveland Manchanda E, Couillard C, Sivashanker K. Inequity in crisis standards of care. N. Engl. J. Med. 2020;383:e16. doi: 10.1056/NEJMp2011359. [DOI] [PubMed] [Google Scholar]
- 157.Schmidt H, Roberts DE, Eneanya ND. Rationing, racism and justice: advancing the debate around ‘colourblind’ COVID-19 ventilator allocation. J. Med. Ethics. 2021 doi: 10.1136/medethics-2020-106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rappoport N, et al. Comparing ethnicity-specific reference intervals for clinical laboratory tests from EHR Data. J. Appl. Lab. Med. 2018;3:366–377. doi: 10.1373/jalm.2018.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huria T, et al. Reported sources of health inequities in indigenous peoples with chronic kidney disease: a systematic review of quantitative studies. BMC Public Health. 2021;21:1447. doi: 10.1186/s12889-021-11180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Elwyn G, et al. Shared decision making: a model for clinical practice. J. Gen. Intern. Med. 2012;27:1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med. Ethics. 2017;18:19. doi: 10.1186/s12910-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roberts DE. Abolish race correction. Lancet. 2021;397:17–18. doi: 10.1016/S0140-6736(20)32716-1. [DOI] [PubMed] [Google Scholar]
- 163.Norris KC, Eneanya ND, Boulware LE. Removal of race from estimates of kidney function: first, do no harm. JAMA. 2021;325:135–137. doi: 10.1001/jama.2020.23373. [DOI] [PubMed] [Google Scholar]
- 164.Diao JA, et al. Clinical implications of removing race from estimates of kidney function. JAMA. 2021;325:184–186. doi: 10.1001/jama.2020.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shin JI, et al. The FDA metformin label change and racial and sex disparities in metformin prescription among patients with CKD. J. Am. Soc. Nephrol. 2020;31:1847–1858. doi: 10.1681/ASN.2019101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Walther CP, Winkelmayer WC, Navaneethan SD. Black race coefficient in GFR estimation and diabetes medications in CKD: national estimates. J. Am. Soc. Nephrol. 2021;32:1319–1321. doi: 10.1681/ASN.2020121724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sehgal AR. Race and the false precision of glomerular filtration rate estimates. Ann. Intern. Med. 2020;173:1008–1009. doi: 10.7326/M20-4951. [DOI] [PubMed] [Google Scholar]
- 168.Miller WG. Uncertainty in estimated glomerular filtration rate is much larger than the race adjustment term. Clin. Chem. 2021;36:464–471. doi: 10.1093/clinchem/hvab007. [DOI] [PubMed] [Google Scholar]
- 169.Lentine KL, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101:S1–S109. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mandelbrot DA, et al. KDOQI US Commentary on the 2017 KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Am. J. Kidney Dis. 2020;75:299–316. doi: 10.1053/j.ajkd.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 171.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Duggal V, Thomas IC, Montez-Rath ME, Chertow GM, Kurella Tamura M. National Estimates of CKD prevalence and potential impact of estimating glomerular filtration rate without race. J. Am. Soc. Nephrol. 2021;32:1454–1463. doi: 10.1681/ASN.2020121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peralta CA, et al. Race differences in prevalence of chronic kidney disease among young adults using creatinine-based glomerular filtration rate-estimating equations. Nephrol. Dial. Transplant. 2010;25:3934–3939. doi: 10.1093/ndt/gfq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.El-Khoury JM, et al. Is it time to move on? Reexamining race in glomerular filtration rate equations. Clin. Chem. 2021;67:585–591. doi: 10.1093/clinchem/hvaa333. [DOI] [PubMed] [Google Scholar]
- 175.Collins FS. What we do and don’t know about ‘race’, ‘ethnicity’, genetics and health at the dawn of the genome era. Nat. Genet. 2004;36:S13–S15. doi: 10.1038/ng1436. [DOI] [PubMed] [Google Scholar]
- 176.Purnell TS, et al. Reduced racial disparity in kidney transplant outcomes in the United States from 1990 to 2012. J. Am. Soc. Nephrol. 2016;27:2511–2518. doi: 10.1681/ASN.2015030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grubbs V. Precision in GFR reporting: let’s stop playing the race card. Clin. J. Am. Soc. Nephrol. 2020;15:1201–1202. doi: 10.2215/CJN.00690120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Powe NR. Black kidney function matters: use or misuse of race? JAMA. 2020;324:737–738. doi: 10.1001/jama.2020.13378. [DOI] [PubMed] [Google Scholar]
- 179.Boulware LE, Purnell TS, Mohottige D. Systemic kidney transplant inequities for black individuals: examining the contribution of racialized kidney function estimating equations. JAMA Netw. Open. 2021;4:e2034630. doi: 10.1001/jamanetworkopen.2020.34630. [DOI] [PubMed] [Google Scholar]
- 180.Delgado C, et al. Reassessing the inclusion of race in diagnosing kidney diseases: an interim report from the NKF-ASN task force. J. Am. Soc. Neprhol. 2021;32:1305–1317. doi: 10.1681/ASN.2021010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Delgado C, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J. Am. Soc. Nephrol. 2021 doi: 10.1681/asn.2021070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Inker LA, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mendu ML, et al. Measuring quality in kidney care: an evaluation of existing quality metrics and approach to facilitating improvements in care delivery. J. Am. Soc. Nephrol. 2020;31:602–614. doi: 10.1681/ASN.2019090869. [DOI] [PMC free article] [PubMed] [Google Scholar]