Abstract
Background and Objectives
To assess whether plasma neurofilament light chain (NfL) levels are elevated before amyotrophic lateral sclerosis (ALS) diagnosis and to evaluate whether prediagnostic NfL levels are associated with metabolic alterations.
Methods
We conducted a matched case–control study nested in 3 large prospective US cohorts (the Nurses' Health Study, the Health Professionals Follow-up Study, and the Multiethnic Cohort Study) and identified 84 individuals who developed ALS during follow-up and had available plasma samples prior to disease diagnosis. For each ALS case, we randomly selected controls from those who were alive at the time of the case diagnosis and matched on birth year, sex, race/ethnicity, fasting status, cohort, and time of blood draw. We measured NfL in the plasma samples and used conditional logistic regression to estimate rate ratios (RRs) and 95% confidence intervals (CIs) for ALS, adjusting for body mass index, smoking, physical activity, and urate levels.
Results
Higher NfL levels were associated with a higher ALS risk in plasma samples collected within 5 years of the ALS diagnosis (RR per 1 SD increase 2.68, 95% CI 1.18–6.08), but not in samples collected further away from the diagnosis (RR per 1 SD increase 1.16, 95% CI 0.78–1.73). A total of 21 metabolites were correlated with prediagnostic NfL levels in ALS cases (p < 0.05), but none of these remained significant after multiple comparison adjustments.
Discussion
Plasma NfL levels were elevated in prediagnostic ALS cases, indicating that NfL may be a useful biomarker already in the earliest stages of the disease.
Classification of Evidence
This study provides Class II evidence that plasma NfL levels are elevated in prediagnostic ALS.
Amyotrophic lateral sclerosis (ALS) is a progressive adult-onset neurodegenerative disease whose etiology is unknown. Despite efforts to create diagnostic criteria that increase the sensitivity and reduce time to diagnosis of the disease, the average time from the first symptom until diagnosis is still 12 months, a figure that has remained similar over the last decade.1 Neurofilament light chain (NfL), a sensitive and specific marker of neuroaxonal damage,2 has consistently been reported to be increased in patients with ALS,3 suggesting that it could be a useful biomarker in ALS. However, most of these studies included patients well into the disease course, making it difficult to determine whether NfL is a reliable marker early in the disease. In a study of asymptomatic individuals with ALS-associated gene mutations, NfL levels were elevated 12 months before the first clinical symptoms,4 suggesting that it is a useful marker for familial ALS. It remains unclear whether NfL is also a useful marker in a general ALS population. Prospective cohort studies with prediagnostic blood samples of patients with ALS are ideal to evaluate early biomarkers, but they are difficult to conduct due to the low incidence of the disease.
We conducted a matched case–control study including participants from 3 large prospective cohorts to determine whether plasma NfL concentrations were elevated before the diagnosis of the disease and examined whether elevation in prediagnostic NfL levels, which could be a marker of the biological onset of ALS, were associated with metabolic alterations using measurements of over 400 metabolites.5
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
All the studies included were reviewed and approved by the institutional review board representing the institution where each study was conducted. All participants provided written informed consent.
Study Populations
We included participants from 3 cohort studies: The Nurses' Health Study (NHS), the Health Professionals Follow-up Study (HPFS), and the Multiethnic Cohort Study (MEC). Detailed descriptions of each cohort have been published previously. The NHS began in 1976 with 121,700 female nurses aged 30–55 years at baseline.6 Blood samples were collected from 32,826 women between 1989 and 1990. A total of 39 nurses were identified with ALS between blood collection and end of follow-up (December 2010). Among these cases, 36 had blood samples available for this study. The HPFS started in 1986 and included 51,529 male health professionals aged 40–75 years at baseline.7 Blood samples were collected from 18,018 men between 1993 and 1995. A total of 26 men developed ALS between blood draw and end of follow-up (December 2010). The participants in these 2 cohorts are followed up biennially with questionnaires on medical history and health-related behavior. The MEC cohort study began in 1993. It comprised 96,810 men and 118,441 women aged 45–75 years at baseline.8 At baseline, the participants were asked to complete a lifestyle and disease history questionnaire, and have since then completed follow-up questionnaires every 5 years. A total of 67,594 members of the cohort have provided a blood sample, which were mostly collected between 2001 and 2006. A total of 31 individuals with available blood samples developed ALS between blood sample collection and end of follow-up (December 2012).
Endpoint Definition
We asked participants in HPFS and NHS to report whether they had received a diagnosis of ALS on the questionnaires administered during follow-up, and requested permission from the participant, or from a family member if the participant was deceased at the time of writing, to contact the neurologist and obtain a copy of the medical records. The neurologist was asked to complete a questionnaire on the certainty of the diagnosis (definite, probable, or possible). A neurologist with clinical experience in ALS confirmed the diagnoses after reviewing the medical records. We included patients with ALS with a definite or probable diagnosis. If we were unable to obtain a copy of the medical record or the neurologist's questionnaire to confirm the ALS diagnosis, only patients with an ALS diagnosis specifically listed on the death certificate were included.
For MEC, we identified patients with ALS by searching the National Death Index. Participants with the code 335.2 (motor neuron disease) according to the ICD-9 listed as the underlying or contributing cause of death were considered to have had ALS. In a validation study, ALS was the primary diagnosis in 90% of the individuals for whom code 335.2 was listed as the cause or contributory cause of death.9 We assigned the date of disease diagnosis to 3 years before the date of death for participants in MEC, based on median survival in patients with ALS.10
In these 3 cohort studies, we identified a total of 84 ALS cases with available prediagnostic blood samples for this study. In addition, we identified 9 ALS cases with blood samples collected shortly after ALS diagnosis. For each of the cases, we randomly selected 1 or 2 controls (depending on available blood samples) from those who were alive at the time of the case diagnosis and matched on birth year (±1 year), sex, ethnicity, cohort, fasting status, and time of blood draw.
Assessment of NfL
Pairs and triplets of samples (from 1 case and its matched controls) were handled identically and assayed in the same batch. The order of the samples within each case–control pair/triplet was arranged at random to make sure that the assays were conducted without knowledge of the case or control status.
Concentrations of NfL were determined in duplicate at the University Hospital Basel using ultrasensitive single-molecule array (Simoa) assays. At the start of the study (2018), 157 plasma samples from NHS and HPFS were available, and these samples were analyzed using Simoa Homebrew Assay (UmanDiagnostics), as previously described.11 A total of 114 additional samples, including all of the 93 samples from MEC, were added to the study in 2020 and were analyzed using the commercially available NF-Light Advantage Kit (Quanterix). NfL concentrations determined by the 2 methods are highly correlated (r2 = 0.96, p < 0.0001). CSF and plasma NfL levels are highly correlated.2 NfL is a sensitive marker of neurodegeneration in ALS, and could in a longitudinal study discriminate patients with ALS from patients with other neurodegenerative conditions, including differential diagnosis of ALS, with high sensitivity and specificity.12 CSF may discriminate patients with ALS from controls with higher sensitivity and specificity compared to serum and plasma levels.13
Assessment of Covariates
Information on body mass index (BMI), smoking, and physical activity was collected from questionnaires in each cohort. We used the nearest covariate data collected before or at the time of blood draw. Only a few participants had missing values for covariates included in the statistical models. Specifically, 4 cases (4.3%) and 8 controls (4.5%) had missing values for physical activity, while 3 cases (3.2%) and 3 controls (1.7%) had missing values for smoking. We imputed missing values using single imputation with the most common category in individuals with the same case–control status. This was done to ensure that all statistical models included the same number of participants. Plasma urate levels were measured via a colorimetric enzyme assay on the Roche P Modular system (Roche Diagnostics), as previously described.14
Statistical Analyses
We log-transformed NfL and metabolite levels to improve normality and standardized the levels (mean 0, SD 1) based on the distribution among the controls of the same cohort. This was done to account for potential differences in levels as a result of the processing methods of blood samples in each cohort. For NfL, we also standardized the levels within assay to account for possible differences in the 2 assays used to determine NfL levels.
We used conditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between NfL levels and ALS risk. Because the controls in the study were selected using risk-set sampling (i.e., the controls were at risk of becoming a case at the time the actual case developed ALS), the ORs estimate incidence rate ratios.15 We modeled NfL levels as a continuous variable (per 1 SD increase in controls) to maximize power if linear and as a categorical variable (tertiles) to explore possible nonlinear associations. For the categorical analyses, we categorized the participants into cohort and assay-specific tertiles based on the distribution among the controls. To test for a linear trend across the tertiles, we assigned the median value to each tertile and modeled this as a continuous variable. To evaluate the influence of potential confounders, we used multivariable models including BMI (continuous), smoking status (categorical: never smoker, past smoker, current smoker), physical activity (categorical: tertiles of metabolic equivalent hours of total physical activity [NHS, HPFS]; tertiles of the amount of moderate and vigorous physical activity [MEC]), and plasma urate levels (continuously). To evaluate whether the association between NfL levels and ALS varied with time to disease diagnosis, we repeated the analyses in predefined strata (<5 years and ≥ 5 years to ALS diagnosis). Only patients with prediagnostic samples were included in the main analyses. In addition, we estimated the ratio of NfL levels in each case–control pair/triplet and plotted these according to time to ALS diagnosis using a locally estimated scatterplot smoothing (LOESS) curve. For the triplets, we calculated the ratio using the mean of the 2 matched controls.
We also evaluated whether NfL levels were associated with changes in the metabolome in ALS cases with blood samples collected within 5 years of disease diagnosis, as NfL levels during this phase are more likely to reflect subclinical disease activity than levels assessed long before the first symptoms.5 To evaluate whether specific metabolites were associated with NfL levels in patients with ALS, we used linear regression models, modeling NfL as the dependent variable and metabolite levels as the independent variable, adjusting for age and sex.
We adjusted for multiple comparisons using the false discovery rate (FDR) approach. Specifically, we used the Benjamini-Yekutieli procedure that accounts for correlations between predictors,16 as implemented in the R Stats package. All analyses were conducted using SAS, version 9.4 software (SAS Institute), and R, version 3.6.0 (the R Foundation). Figures were made using the ggplot2 and ggpol R packages. p Values were considered significant at values <0.05, and all tests were 2-sided.
Data Availability
The datasets analyzed in the current study are not publicly available because of restricted access, but further information about the datasets is available from the corresponding author on request.
Results
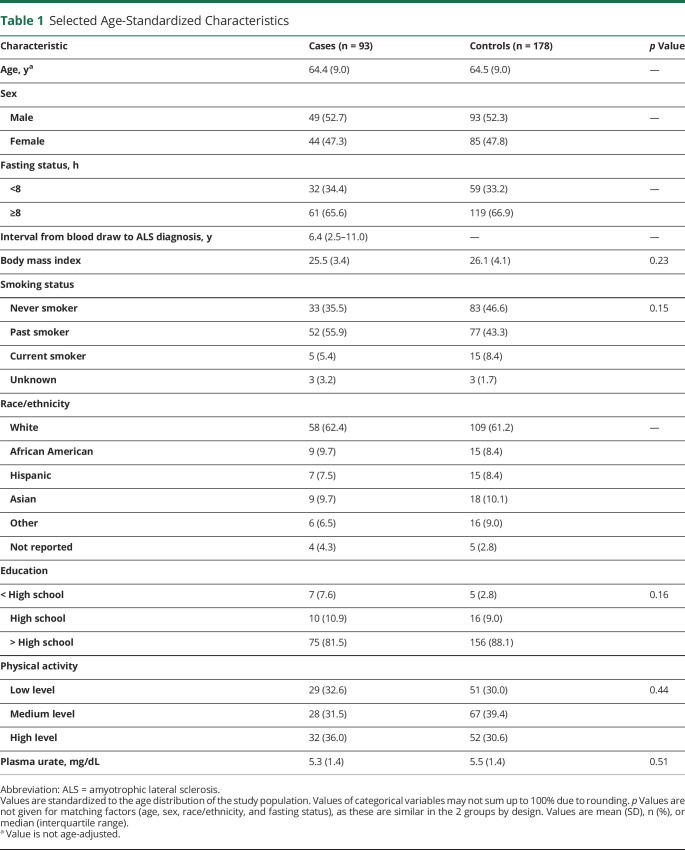
We identified a total of 93 individuals who developed ALS among the 118,437 participants who provided blood samples in these 3 cohorts. Among these, 84 individuals had available prediagnostic samples. The baseline characteristics were similarly distributed in cases and controls (Table 1), but the cases had slightly lower BMI, which is consistent with what has previously been reported,17,18 and were more likely to have been smokers. Fewer cases had higher educational levels than high school compared to the controls. Overall, the NfL concentrations were lower in samples analyzed with NF-Light Kit than the Simoa Homebrew Assay (Figure 1), as illustrated by the lower median levels comparing the assays among the healthy controls, which is consistent with other studies.11,19
Table 1.
Selected Age-Standardized Characteristics
Figure 1. Box Plots and Dot Plots of Neurofilament Light Chain (NfL) Levels in Prediagnostic Amyotrophic Lateral Sclerosis (ALS) Cases and Matched Controls as Quantified by 2 Methods.
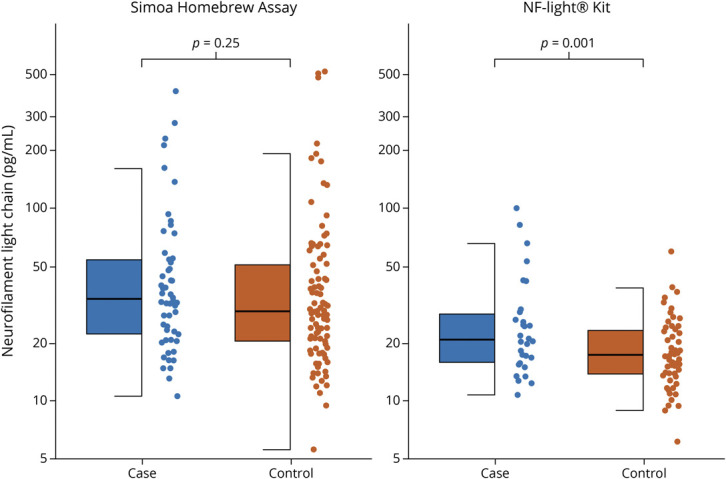
The figure illustrates levels of NfL in ALS cases and matched controls. Log-transformed NfL levels were compared using linear mixed-effects models with random effects for matched pairs/triplets. The lower and upper hinges correspond to the first and third quartiles. The upper whisker extends to the largest value at most 1.5 * interquartile range from the hinge, while the lower whisker extends to the smallest value no further than 1.5 * interquartile range of the hinge.
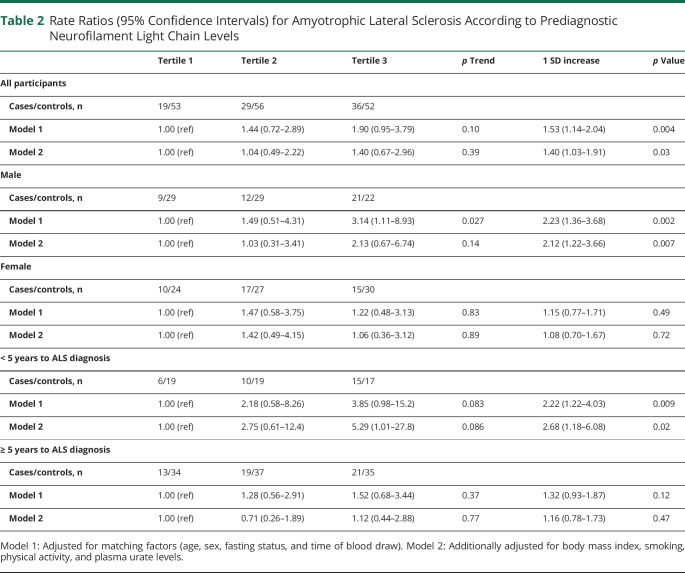
Higher prediagnostic NfL levels were associated with a higher ALS risk in analyses adjusted for age, sex, and matching factors in continuous and categorical analyses (Table 2). The results remained similar in a multivariable model further adjusted for BMI, smoking, physical activity, and urate levels. In the fully adjusted multivariable model, the rate ratio (RR) for ALS per 1 SD increase in prediagnostic NfL was 1.40 (95% CI 1.03–1.91, p = 0.03). The RR comparing the top vs lowest tertiles and the p for trend across the tertiles did not reach statistical significance.
Table 2.
Rate Ratios (95% Confidence Intervals) for Amyotrophic Lateral Sclerosis According to Prediagnostic Neurofilament Light Chain Levels
In analyses stratified on time to ALS diagnosis, prediagnostic NfL levels were only significantly associated with ALS risk in the analysis restricted to those with shorter interval (0 to <5 years) between blood draw and diagnosis (Table 2). The multivariable-adjusted RR for ALS per 1 SD increase in prediagnostic NfL in this group was 2.68 (95% CI 1.18–6.08, p = 0.02), as compared to 1.16 (95% CI 0.78–1.73, p = 0.47) in the group with an interval of 5 years or longer. The RRs comparing the top vs lowest quartiles and the p for trend did not reach statistical significance. When we plotted the ratio of NfL in matched case–control pairs/triplets according to time to ALS diagnosis, we observed that the levels were similar in cases and controls until 1–2 years before the diagnosis (Figure 2). In the 2 years before the diagnosis, the ratio was positive (i.e., NfL levels were higher in the cases compared to matched controls) in 9 of the 13 pairs/triplets (69.2%). Among ALS cases with postdiagnostic samples (n = 9), NfL levels were higher in cases than in controls in 7 of the 9 pairs/triplets (77.8%), but the risk estimates did not reach statistical significance (RR per 1 SD increase in NfL: 3.04, 95% CI 0.95–9.79, p = 0.06). In a sensitivity analysis, the estimates remained similar when restricting the analysis to participants with no missing values for covariates. The multivariable-adjusted RR for ALS per 1 SD increase in prediagnostic NfL levels within 5 years of ALS diagnosis was 2.39 (95% CI 1.08–5.29; p = 0.03).
Figure 2. Ratio of Neurofilament Light Chain (NfL) Levels in Amyotrophic Lateral Sclerosis (ALS) Cases and Matched Controls According to Time to ALS Diagnosis.
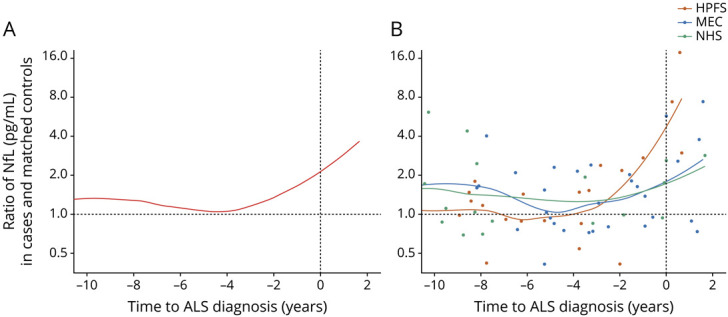
The figure illustrates the relative difference (ratio) in NfL levels in ALS cases and matched controls. The locally estimated scatterplot smoothing (LOESS) curve illustrates the changes in the ratio over time. Each point corresponds to 1 case–control pair/triplet. (A) The LOESS curve was estimated from all case–control pairs/triplets. (B) A LOESS curve was estimated for each cohort separately. HPFS = Health Professionals Follow-up Study; MEC = Multiethnic Cohort Study; NHS = Nurses' Health Study.
The risk estimates were higher in male than in female patients, but the proportion of samples collected within 5 years of ALS diagnosis was considerably lower in female (24.4%) than in male (45.9%) patients. Similarly, there was only a significant association between NfL and ALS in samples assayed with the NfL kit (Figure 1), but the proportion of samples collected within 5 years of ALS diagnosis was 70.0%, as compared to 14.8% for the Simoa Homebrew assay.
In analyses comparing NfL levels with the levels of 404 metabolites in ALS cases, we detected 21 metabolites that were nominally significantly correlated with NfL. None of these associations retained significance after we accounted for multiple comparisons.
Discussion
In this study, we found that NfL levels were increased in ALS cases compared to their matched controls before the diagnosis of the disease. The levels increased closer to time of diagnosis, in particular in the last 12–24 months. These results suggest that NfL is a useful biomarker already in the earliest stages of the disease.
Few studies have evaluated the role of NfL in prediagnostic ALS. One study followed asymptomatic carriers at risk of developing ALS over time, and found that, among those who phenoconverted (n = 10), NfL levels appeared to increase approximately 12 months before the earliest clinical symptoms or signs of the disease.4 Later analyses in the same cohort of patients suggest that the presymptomatic NfL elevation may depend on genotype, as changes occurred as early as 3.5 years before the disease onset in a patient with a C9ORF72 repeat expansion, but only 6–12 months before the onset in patients with an SOD1 A4V mutation.20 Due to the low number of patients with each genetic mutation, these results may be prone to random variation. In a separate study including asymptomatic carriers, NfL levels were not significantly different from controls.21 However, most samples in this study were collected more than 24 months before clinical onset, which may have been too early to detect any differences. Furthermore, age at onset was imputed based on the parental age at ALS onset, which may have led to misclassification of the actual time to onset. There are no studies on NfL in prediagnostic sporadic ALS, but a recent study found a significant increase in serum neurofilament heavy chain (NfH) up to 18 months before diagnosis.22 Ten of the patients in this study had available presymptomatic blood samples, and 5 of these had NfH levels above a predefined cutoff, which discriminated patients with ALS from ALS mimics in a previous study.23 NfH was a less sensitive marker than NfL during the presymptomatic phase in asymptomatic carriers,20 suggesting that results from studies on NfH and NfL may not be directly comparable. Our findings indicate that NfL levels are elevated shortly before the clinical onset, making it a useful biomarker at the earliest disease stages of ALS. This is consistent with the findings in a larger study of patients with ALS, where serum and CSF NfL levels were consistently increased in patients early in their disease course (≤6 months from symptom onset) and could discriminate patients with ALS from those with other neurologic diseases.24
In our study, we found that elevated prediagnostic NfL levels, which likely reflect early disease processes in ALS, were not associated with a distinct metabolomic signature. This is consistent with the results from our previous metabolomics study suggesting ALS is not preceded by distinct metabolic alterations, but a broad, poorly defined metabolic dysregulation.5 We observed that 21 metabolites correlated significantly with NfL levels in the 5 years before ALS diagnosis. None of the metabolites retained significance after we accounted for multiple comparisons, which likely reflects the low statistical power in these analyses, but could also reflect that few metabolic markers are consistently elevated in the years before ALS diagnosis, even after the disease processes have started (i.e., the biological onset of the disease).
Several neurologic diseases, including Alzheimer disease (AD),25 Parkinson disease (PD),26 and multiple sclerosis (MS),27 appear to have a preclinical phase lasting several years before the first clinical symptoms become apparent. This can make it challenging to conduct etiologic research, as an exposure must precede the outcome to be causal,28 and a long preclinical phase makes it difficult to separate risk factors from consequences of presymptomatic disease processes. In our study, we found no association between NfL and ALS in samples obtained 5 years or longer before ALS diagnosis and observed that the biomarker levels only appeared to increase 12–24 months before the diagnosis. This suggests that ALS is characterized by a shorter preclinical phase than AD, PD, and MS. Still, the preclinical phase may be longer than suggested by our results, as our analyses were based on time of diagnosis, and symptom onset may start some time before the disease is diagnosed.
Our study has several strengths. The nested case–control design makes it unlikely that there were any systematic differences between cases and controls in their source population and procedures of blood collection and processing. As the blood samples from cases and controls were analyzed at the same time, blindly, and in random order, it is unlikely that there were any artefactual differences in the measured NfL levels. Our study also has some limitations to consider. While we included participants from 3 large cohort studies, we only identified 84 patients with ALS who had prediagnostic blood samples available for our study, which may have limited the statistical power in some analyses and made our estimates more prone to random variation. This challenge is inherent to the difficulty of conducting prospective studies of a rare disease like ALS. For MEC, we did not have access to clinical information and relied on death certificates to identify individuals who developed ALS. This may have led to misclassification of ALS in some participants, but use of death certificate appears to be an adequate, although not ideal, tool to capture patients with ALS in epidemiologic studies.29 Furthermore, because of the lack of clinical information, we imputed time of ALS diagnosis among participants in MEC, which likely led to misclassification of the time of the onset in some individuals. Still, our results are consistent with previous studies on neurofilaments in prediagnostic ALS. We imputed missing values for covariates to ensure that all models included the same number of participants, which could lead to bias if data are not missing at random. The results remained similar in a sensitivity analysis restricted to individuals without missing data. We did not have clinical and genetic information on the ALS cases to distinguish ALS subtypes and therefore could not separate sporadic from familial cases in our study. Lastly, as is inherent to any observational study, we cannot exclude the possibility that the results may be affected by residual or unmeasured confounding that we could not account for.
Glossary
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- BMI
body mass index
- CI
confidence interval
- FDR
false discovery rate
- HPFS
Health Professionals Follow-up Study
- ICD-9
International Classification of Diseases, Ninth Revision
- LOESS
locally estimated scatterplot smoothing
- MEC
Multiethnic Cohort Study
- MS
multiple sclerosis
- NfH
neurofilament heavy chain
- NfL
neurofilament light chain
- NHS
Nurses' Health Study
- OR
odds ratio
- PD
Parkinson disease
- RR
rate ratio
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
Funded by a grant from an ALS Biomarker Consortium with funding partners including the ALS Association, ALS Finding a Cure, CreATe, MDA, and Packard Center for ALS Research. The NHS is funded by the NIH through grants UM1 CA186107 and R01 CA49449. The HPFS cohort is funded by the NIH through grant U01 CA167552. The MEC cohort is funded by the NIH through U01 CA164973. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
K. Bjornevik reports research grants from the ALS Association and ALS Finding a Cure. É.J. O'Reilly, S. Molsberry, L. N. Kolonel, and L. Le Marchand report no disclosures. S. Paganoni reports grants from the ALS Association, Amylyx Pharmaceuticals, Revalesio Corporation, ALS Finding a Cure, the American Academy of Neurology, the Salah Foundation, and the Spastic Paraplegia Foundation. M.A. Schwarzschild and P. Benkert report no disclosures. J. Kuhle received speaker fees, research support, travel support, and/or served on advisory boards for Swiss MS Society, Swiss National Research Foundation, University of Basel, Progressive MS Alliance, Bayer, Biogen, Celgene, Merck, Novartis, Octave Bioscience, Roche, and Sanofi. A. Ascherio reports no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Mitchell JD, Callagher P, Gardham J, et al. Timelines in the diagnostic evaluation of people with suspected amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND): a 20-year review: can we do better? Amyotroph Lateral Scler. 2010;11(16):537-541. [DOI] [PubMed] [Google Scholar]
- 2.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(110):577-589. [DOI] [PubMed] [Google Scholar]
- 3.Xu Z, Henderson RD, David M, McCombe PA. Neurofilaments as biomarkers for amyotrophic lateral sclerosis: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0164625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol. 2018;84(1):130-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjornevik K, Zhang Z, O'Reilly EJ, et al. Prediagnostic plasma metabolomics and the risk of amyotrophic lateral sclerosis. Neurology. 2019;92(118):e2089-e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three Nurses' Health Studies. Am J Public Health. 2016;106(19):1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(18765):464-468. [DOI] [PubMed] [Google Scholar]
- 8.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(1):346-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisskopf MG, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol. 2004;160(1):26-33. [DOI] [PubMed] [Google Scholar]
- 10.Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract. 2013;3(14):313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(16):857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verde F, Steinacker P, Weishaupt JH, et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(12:157-164. [DOI] [PubMed] [Google Scholar]
- 13.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;85(110):921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly EJ, Bjornevik K, Schwarzschild MA, et al. Pre-diagnostic plasma urate and the risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(13-4):194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knol MJ, Vandenbroucke JP, Scott P, Egger M. What do case-control studies estimate? Survey of methods and assumptions in published case-control research. Am J Epidemiol. 2008;168(19):1073-1081. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(14):1165-1188. [Google Scholar]
- 17.Nakken O, Meyer HE, Stigum H, Holmoy T. High BMI is associated with low ALS risk: a population-based study. Neurology. 2019;93(15):e424-e432. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly EJ, Wang M, Adami HO, et al. Prediagnostic body size and risk of amyotrophic lateral sclerosis death in 10 studies. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(15-6):396-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manouchehrinia A, Stridh P, Khademi M, et al. Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology. 2020;94(123):e2457-e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benatar M, Wuu J, Lombardi V, et al. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(17-8):538-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weydt P, Oeckl P, Huss A, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. 2016;79(1):152-158. [DOI] [PubMed] [Google Scholar]
- 22.De Schaepdryver M, Goossens J, De Meyer S, et al. Serum neurofilament heavy chains as early marker of motor neuron degeneration. Ann Clin Transl Neurol. 2019;6(110):1971-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Schaepdryver M, Jeromin A, Gille B, et al. Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(14):367-373. [DOI] [PubMed] [Google Scholar]
- 24.Feneberg E, Oeckl P, Steinacker P, et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology. 2018;90(1):e22-e30. [DOI] [PubMed] [Google Scholar]
- 25.Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25(12):277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386(9996):896-912. [DOI] [PubMed] [Google Scholar]
- 27.Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2019;77(1):58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(15):295-300. [PMC free article] [PubMed] [Google Scholar]
- 29.Kioumourtzoglou MA, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3-4):224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are not publicly available because of restricted access, but further information about the datasets is available from the corresponding author on request.