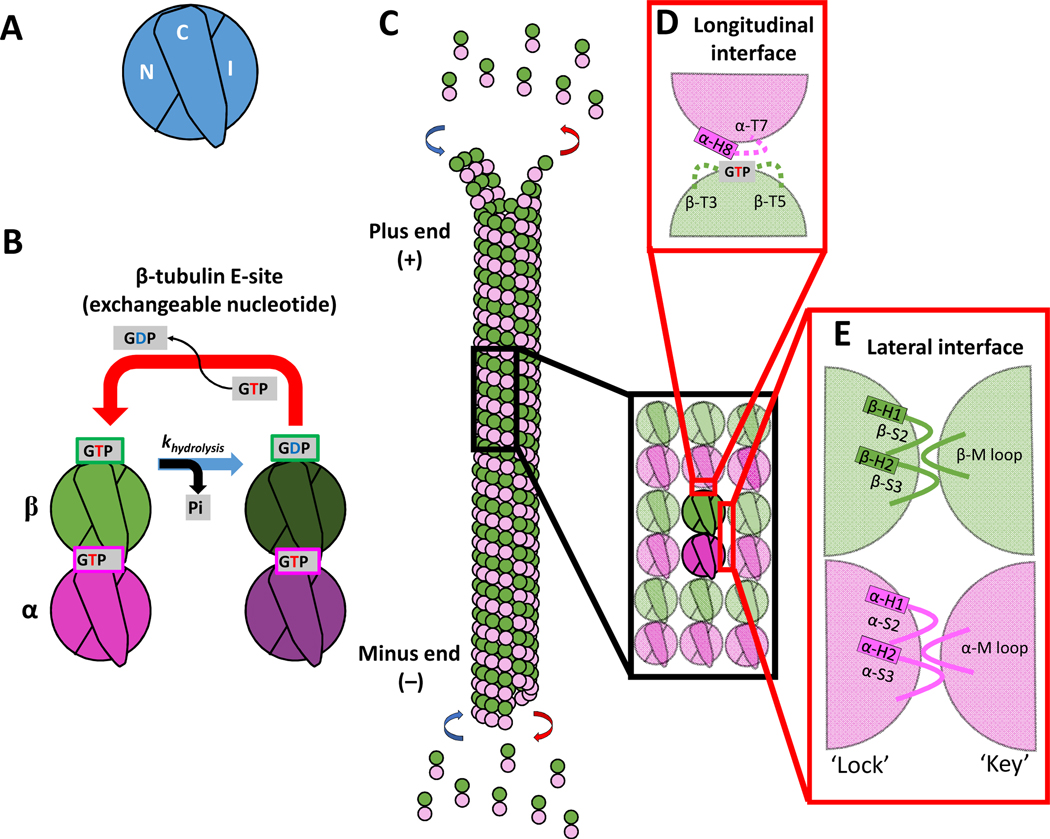
Figure 1. Domains of tubulin and the interdimer contacts involved in microtubule growth.
(A) Three domains that make up a tubulin monomer: the N-terminal domain containing the nucleotide pocket (N), the intermediate domain made from the globular region of the protein (I), and the C-terminal tail (C) facing the outside of the microtubule.
(B) The exchangeable GTP binding site (E-site), where GTP hydrolysis and nucleotide exchange occurs, is located in the N-domain of β-tubulin. Tubulin can be in either the GTP or GDP state.
(C) Schematic of a microtubule growing by tubulin addition at the plus- and minus-ends.
(D) Longitudinal (top-to-bottom) dimer-dimer contacts involve interactions between the N-domain of β-tubulin of one dimer and the I-domain of α-tubulin of a second dimer. This longitudinal interaction between dimers forms a nucleotide pocket around the exposed GTP on the β-tubulin.
(E) Lateral (side-to-side) dimer-dimer contacts form a lock-and key pocket between the I-domain of one tubulin dimer and the N-domain of the adjacent dimer.