Abstract
Impaired glucose regulation (IGR) is common world-wide, and is correlated with Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) the virus that causes Coronavirus disease 2019 (COVID-19). However, no systematic reviews are available on the topic, and little is known about the strength of the evidence underlying published associations. The current systematic review identified consistent, reproducible associations but several limitations were observed including: (1) a consistent lack of robust confounder adjustment for risk factors collected prior to infection; (2) lack of data on insulin resistance or glycemia measures [Hemoglobin A1c (HbA1c) or glucose]; (3) few studies considering insulin resistance, glucose or HbA1c values in the clinically normal range as a predictor of SARS-CoV-2 risk; (4) few studies assessed the role of IGR as a risk factor for infection among initially uninfected samples; (5) a paucity of population-based data considering SARS-CoV-2 as a risk factor for the onset of IGR. While diabetes status is a clear predictor of poor prognosis following a SARS-CoV-2 infection, causal conclusions are limited. It is uncertain whether interventions targeting dysglycemia to improve SARS-CoV-2 outcomes have potential to be effective, or if risk assessment should include biomarkers of diabetes risk (ie, insulin and glucose or HbA1c) among diabetes-free individuals. Future studies with robust risk factor data collection, among population-based samples with pre-pandemic assessments will be important to inform these questions.
Abbreviations: IGR, Impaired glucose regulation; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; COVID-19, Coronavirus disease 2019; T2DM, Type 2 Diabetes Mellitus; HbA1c, Hemoglobin A1c; ACE-2, Angiotensin Converting Enzyme-2; TMPRSS2, Transmembrane serine protease 2; T1DM, Type 1 Diabetes Mellitus
INTRODUCTION
Diabetes Mellitus is believed to be an important risk factor predisposing to Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes Coronavirus disease 2019 (COVID-19) infection as well as severity of infection and risk for hospitalization and mortality. Interestingly, less is known about the relationships between non-diabetic impaired glucose regulation (for the purposes of this review, prediabetes, and insulin resistance) as a potential risk factor for SARS-CoV-2 infection. For example, Rosenthal et al reported that 29.9% of inpatients with a diagnosis of COVID-19 had no co-morbidities using the Charlson-Deyo comorbidity index suggesting the importance of phenotypic characteristics other than comorbidity burden.1
One of the key factors in deciphering the above conundrum is to view Type 2 Diabetes Mellitus (T2DM) as a continuum of glucose dysregulation and not merely dichotomously by hyperglycemia exceeding thresholds (largely identified vis-à-vis their prediction of microvascular outcomes).2 Tabak et al observed a modest linear increase in fasting and post prandial glucose, followed by a sharp rise in the aforementioned levels and a steeper decrease in HOMA insulin sensitivity in the 5 years prior to diagnosis of T2DM, suggesting that individuals with prediabetes are already in the accelerated phase, trending towards overt diabetes.3 The relative risk of cardiovascular events was 1.33 (95% CI:1.06-1.67) for impaired fasting glucose and 1.58 (95% CI: 1.19-2.10) for impaired glucose tolerance.4 The current guidelines in the screening, diagnosis, and management of cardiometabolic disease have shifted towards prioritizing risk factors of preclinical entities such as insulin resistance and prediabetes. For instance, the United States Preventive Services Task Force updated their recommendations in 2015 to include screening for diabetes among those ages 40-70 years who are overweight or obese which was previously limited to asymptomatic individuals with hypertension.5 Similarly, the American Association of Clinical Endocrinologists introduced the dysglycemia based chronic disease model (DBCD) to include stage I of insulin resistance, stage II of biochemical risk or prediabetes, stage III of T2DM, and stage IV vascular complications, to encourage aggressive cardiovascular management as early as stage I.6 Although uncommon, the microvascular complications of T2DM, specifically retinopathy has been reported in the impaired glucose regulation phase, before diabetes is diagnosed.7 Thefore, considering impaired glucose regulation as a continuum in relation to SARS-CoV-2 outcomes could add great value to risk prediction as SARS-CoV-2 infection will likely become endemic.8
Beyond the role of IGR as a predictor of SARS-CoV-2 infection and poor outcomes post-infection, there is preliminary evidence that SARS-CoV-2 infection itself might be a risk factor for impaired glucose regulation and subsequent diabetes development among diabetes-free individuals. There is a strong rationale to believe this is possible based on the broader literature linking infections to adverse metabolic outcomes including insulin resistance,9, 10, 11 glucose intolerance12 and diabetes development.2 , 13, 14, 15, 16
In this review, we systematically search the literature for published data on the interplay between impaired glucose regulation, diabetes mellitus and SARS-CoV-2. We summarize key findings and discuss a variety of methodological issues and their implications for understanding whether metabolic parameters predict risk of SARS-CoV-2 infection and poor COVID-19 outcomes, as well as the evidence that SARS-CoV-2 induces metabolic abnormalities increasing diabetes risk. We additionally summarize emerging concepts related to the biological plausibility of the relationships.
LITERATURE SEARCH STRATEGY
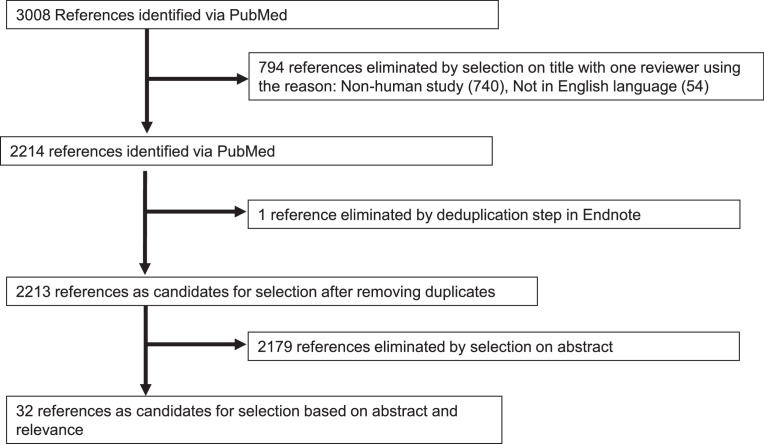
To assess the evidence from population-based and clinically-based human studies, we searched PubMed for literature published on impaired glucose regulation, diabetes, SARS-CoV-2, and COVID-19 between December 2019 and June 2021. The search strategy is summarized in Table I. Initially 3,008 publications were identified. A primary reviewer SR removed duplicate publications and reviewed titles for inclusion. Ambiguous inclusion decisions were discussed by both authors (SR and RTD) to determine inclusion and/or exclusion. Review articles (systematic reviews, meta-analysis), case reports, studies that were done exclusively in children and adolescence or in pregnancy and studies that did not report a measure of association pertinent to the specific search criteria were excluded. The final selection flow is summarized in Fig 1 and 32 articles assessing metabolic parameters and SARS-CoV-2 infection were included.
Table I.
PUBMED search equation
| 1. severe acute respiratory syndrome coronavirus 2 |
| "sars cov 2"[MeSH Terms] OR "sars cov 2"[All Fields] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] |
| 2. SARS COV 2 |
| "sars cov 2"[MeSH Terms] OR "sars cov 2"[All Fields] OR "sars cov 2"[All Fields] |
| 3. COVID 19 |
| "covid 19"[MeSH Terms] |
| 4. Equation: 1 OR 2 OR 3 |
| 5. Prediabetes |
| "prediabetic state"[MeSH Terms] OR ("prediabetic"[All Fields] AND "state"[All Fields]) OR "prediabetic state"[All Fields] OR "prediabetes"[All Fields] OR "prediabetic"[All Fields] OR "prediabetics"[All Fields] |
| 6. Insulin resistance |
| "insulin resistance"[MeSH Terms] OR ("insulin"[All Fields] AND "resistance"[All Fields]) OR "insulin resistance"[All Fields] |
| 7. Glucose |
| "glucose"[MeSH Terms] OR "glucose"[All Fields] OR "glucoses"[All Fields] OR "glucose s"[All Fields] |
| 8.Hemoglobin A1C |
| "glycated hemoglobin a"[MeSH Terms] OR "glycated hemoglobin a"[All Fields] OR ("hemoglobin"[All Fields] AND "a1c"[All Fields]) OR "hemoglobin a1c"[All Fields] |
| 9. Diabetes Mellitus |
| "diabetes mellitus"[MeSH Terms] OR ("diabetes"[All Fields] AND "mellitus"[All Fields]) OR "diabetes mellitus"[All Fields] |
| 10. Equation 5 OR 6 OR 7 OR 8 OR 9 |
| 11. Equation: 4 AND 10 |
Fig 1.
Study selection flow diagram.
METABOLIC PARAMETERS, INFECTION RISK AND SEVERITY
Diabetes Mellitus and Risk of Infection
Nearly all studies identified included only study samples with SARS-CoV-2 infected patients, while only five studies considered samples with and without SARS-CoV-2 infected individuals. Therefore, limited data exist considering the contribution of diabetes status to the risk of becoming SARS-CoV-2 infected. Among studies that do include samples with and without infection, most report on poor SARS-CoV-2 related outcomes rather than the cumulative incidence of infection. Nevertheless, while sparse, the limited data are informative. McGurnaghan et al 17 provide total population level data among 5,463,300 Scotland residents (5.8% with diabetes) and assessed whether diabetes status was associated with critical care unit-treated COVID-19 or mortality. The age and sex adjusted odds ratios for poor COVID outcomes was 2.40[1.82-3.16] among people with T1DM (Type 1 Diabetes Mellitus) and 1.37[1.28-1.47] for people with T2DM (vs diabetes-free individuals). While this study cannot fully address the risk of infection associated with diabetes, it informs the risk for severe infection including mortality among an initially infection-free cohort. In another study among nearly the total population of England, a third of all in-hospital deaths with COVID-19 between March 1 and May 11, 2020, occurred among people with diabetes. Mortality rates were higher among those with diabetes vs those without; T1DM again demonstrated stronger mortality risk than T2DM.18 Similarly, studies from Mexico,19 and Italy20 also reported higher risk of SARS-CoV-2 outcomes (including hospitalization and mortality) among those with vs. without diabetes (Table II).
Table II.
Summary of studies assessing the association between diabetes mellitus and SARS-CoV-2 infection and/or COVID-19 outcomes
| Study | Country | N | Mean Age±SD (range) | Male (%) | Study Design | Diabetes Assessment | Outcome | SARS-CoV-2 Assessment | Study population | Adjustments | Measure of Association (95% Confidence Interval) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population based studies | |||||||||||
| Woolcott et al. 2021 | Mexico | 757,210 | 44 (33–56) | 52% | Cohort study | Self-reported | COVID-19 mortality within 28 days of evaluation | Positive RT-PCR | ≥20 years, viral respiratory disease symptoms, screened for SARS-CoV-2 via the System of Epidemiological Surveillance of Viral Respiratory Disease from Jan-Nov, 2020 | 1,2,5,6a,6c,6d, 6e,6h,19 | HR 1.49 (1.47–1.52)-Overall HR 1.66 (1.58–1.74) Outpatients HR 1.14 (1.12–1.16) Hospitalized |
| Barron et al. 2020 | UK | 61,414,470 | 41±23 | 50% | Cohort study | EHR | COVID-related in-hospital mortality | Positive antigen test or if negative, COVID-19 reported as cause of death | All individuals registered with a general practice in England, alive on Feb 16, 2020 | 1,2,4,7-9 | OR 2·86 (2·58-3·18)-T1DM OR 1·80 (1·75-1·86)-T2DM |
| Dennis et al. 2021 | UK | 19,256 | 67 | 60% | Cohort study | England Surveillance System (CHESS) | In-hospital mortality | Positive RT-PCR of nasopharyngeal and/or oropharyngeal swabs | Hospitalization due to COVID-19 requiring admission to a high dependency unit (HDU) or intensive care unit (ICU) from March- July 2020, excluding pregnancy | 1-5,6(a-h) | HR 1.23, (1.14, 1.32) |
| McGurnaghan et al.2021 | UK | 5,463,300 | NR | NR | Cohort study | National Diabetes Register | Fatal or critical care unit-treated COVID-19 | Positive RT-PCR test, a hospital discharge code for COVID-19, or COVID-19 code on death certificate | Total Scottish population at the start of the pandemic on Mar 1, 2020 | 1,2 | OR 1·39 (1·30–1·49)-Overall OR 2·39 (1·81–3·16)-T1DM OR 1·36 (1·27–1·46)-T2DM |
| Silverii et al.2021 | Italy | 4,969,000 | NR | NR | Cohort study | Italian SARS-CoV-2+ Surveillance System | COVID-19 prevalence by diabetes status | testing method not specified | Total population of Sicily | 1,2 | RR 0.92 (0.79-1.09) |
| 159 | 73±13 | COVID-19 mortality | All SARS-CoV-2 positive cases | 1,2 | RR 4.5 (3.55-5.71)-DM | ||||||
| CDC COVID-19 Response Team | USA | 7,162 | NR | NR | MMWR Report | Documented in case report form | COVID-19 | Laboratory confirmed COVID-19 | Data of COVID-19 cases reported to CDC from 50 states, four U.S. territories and affiliated islands, the District of Columbia, and New York City from Feb–Mar,2020 |
1† | Prevalence n(%) 784, (10.9) |
|
Hospital based studies |
|||||||||||
| Collard et al. 2021 | Netherlands | 1604 | 66±15 | 61% | Cohort study | EHR/ antidiabetic medication | Mortality during first 21 days following admission | Positive COVID-19 PCR or high suspicion based on CT-imaging of the thorax. |
Multi-center, Dutch CovidPredict cohort | 1,2,5,6d,19 | HR 1.93 (1.43-2.62)-≥2 antidiabetic HR 1.25(0.9-1.74)-1 antidiabetic. (Ref- No Diabetes) |
| Altunok et al. 2021 | Turkey | 722 | 57±15 | 51% | Cohort study | NR | Mortality | Positive nasopharyngeal RT-PCR with chest CT findings compatible with COVID-19 pneumonia | Single center, patients with laboratory and chest CT confirmed COVID-19 pneumonia b/w Mar-May, 2020 | None | OR-1.97 (0.71-5.41) |
| Klonoff et al. 2021 | USA | 1544 | 64±16 | 54% | Cohort study | Severe hyperglycemia: BG>13.88 mmol/L | Mortality | COVID-19–positive laboratory test | Multi-center, patients with COVID-19 from Glytec national database from 91 hospitals in 12 states. | 1,2,10,13,15 | Severe hyperglycemia Non-ICU: HR 7.17 (2.62–19.62)-day 2-3 glucose HR 1.46 (0.68-3.14)- glucose on admission ICU: HR 1.40 (0.53–3.69)-day 2-3 glucose HR 3.14 (1.44-6.88)- glucose on admission Ref: BG <7.77 mmol/L (140 mg/dL) |
| Liu et al. 2021 | China | 77 | 64±4 | 62% | Cohort study | HbA1c ≥6.5% | Mortality | Positive RT–PCR | Hospitalized patients with confirmed COVID‐19-identified as severe cases or critically ill cases at admission. | 1,14,18,20,24 | HR 1.57 (1.15–2.15) |
| Liu, Ye et al. 2021 | China | 233 | 64 | 49% | Cohort study | Self-reported/HbA1c on admission ≥ 48 mmol/mol |
Mortality | Two clinical manifestations of COVID-19 and positive RT-PCR nasopharyngeal samples | Single center study, patients admitted from Jan-Apr, 2020 | None | Diabetes: HR 2.64 (1.14-6.11) Hba1c ≥ 6.5% HR 5.80 (1.32, 25.53) Ref: Hba1c 5.7-6.4% FPG ≥ 7.0 HR 12.64 (2.93, 54.48) 5.6-6.9 HR 3.49 (0.73, 16.82) Ref:≤ 5.5 |
| Li et al. 2020 | China | 453 | 61 (49-68) * | 57%-known DM 61%-newly diagnosed | Cohort study | H/O Diabetes, fasting glucose ≥7mmol/L and/or HbA1c ≥6.5% on admission | Mortality | Exposure to confirmed SARS-CoV-2 infection or to Wuhan Huanan seafood market | Patients admitted with laboratory‐confirmed SARS‐Cov‐2 infection from Jan-Mar, 2020. | 1,2,19,47-53 | Newly diagnosed diabetes HR 5.63 (1.22-26) Known diabetes HR 8.76 (1.78-43.2) |
| Sourij et al. 2021 | Austria | 238 | 71±13 | 64% | Cohort study | Diabetes diagnosed according to the Austrian Diabetes Association guidelines. | Mortality | Positive throat swab for SARS‐CoV‐2 | Patients with diabetes hospitalized with COVID-19. | None | OR 1.85 (0.66‐5.16) -T2DM OR 0.46 (0.44‐4.90) -T1DM |
| Mamtani et al. 2021 | USA | 403 | 55 | 68% | Cohort study | Hyperglycemia: [one blood glucose value ≥7.78 mmol/L during hospitalization. Diabetes: HbA1c ≥6.5% | Mortality | Positive PCR for the RdRp and N genes | Single center, COVID-19 patients hospitalized from March-May 2020 | 1,27-32 | Diabetes/no-hyperglycemia OR 5.97 (0.32–111.8) Diabetes/hyperglycemia OR 17.06 (3.46–84.1). (Ref: No Diabetes/No hyperglycemia) |
| Nafakhi et al. 2020 | Iraq | 192 | 60±10 | 43% | Cohort study | HbA1c >6.5%, h/o diabetes, on antidiabetic medication | COVID-19 pneumonia related length of ICU stay, hospital stay, lung injury, in-hospital death | Positive RT-PCR -nasopharyngeal swab and clinical symptoms of COVID-19 along with CT scan to assess pneumonia severity | Single center, patients diagnosed with COVID-19 pneumonia at the outpatient clinic or admitted from Aug-Oct 2020 | 1,20,27,41% | Insulin use OR 0.4(0.3-5)-lung injury Metformin use OR 0.1(0.1–0.6)-in hospital death OR -0.3(0.2–4)- length of ICU stay OR -0.4(0.2–3)-Length of hospital stay DDP-4 OR -0.3(0.2-3)- length of ICU stay |
| Al-Salameh et al. 2021 | France | 433 | 72±14-DM 71±16-non-DM | 55% | Cohort study | EHR, HbA1c ≥48 mmol/mol on admission | Primary endpoint: admission to ICU and/or death. | Positive RT-PCR-nasopharyngeal swab | Single center, patients hospitalized with COVID-19 | 1,2,5,6a,6,c, 6d,6e,6i, 11,12,13, 15,16,17,21,22 | OR 1.12 (0.66-1.90)- Primary endpoint OR: 2.06 (1.09-3.92)-ICU admission HR 0.73 (0.40-1.34)- Mortality |
| Fox et al. 2021 | USA | 355 | 66 ± 14 | 51% | Cohort study | Patients on antidiabetic medication prior to admission | Composite outcome of inpatient mortality, need for renal replacement therapy/hemodialysis, intubation, and vasopressors | Positive RT-PCR-nasopharyngeal swab | Single-center, COVID-19 positive patients, admitted from Mar-Apr 2020 | 1,2,3,15,6b-e,6i-k | OR 1.4 (0.84 to 2.31) |
| Kim et al. 2021 | USA | 2,491 | 62 (50-75) * | 53% | Cohort study | EHR | a) In-hospital mortality b) ICU admission | Resided in a predefined surveillance catchment area; positive SARS-CoV-2 test within 14 days prior to/during hospitalization | Coronavirus Disease 2019-Associated Hospitalization Surveillance Network | a)1,2,3,4,5,6c-h,19,22. b)1,2,3,4,5,6c-h 6l-m,19 | a) Risk ratio 1.19 (1.01–1.40) in-hospital mortality b) Risk ratio: 1.13 (1.03–1.24)-ICU admission |
| Orioli et al. 2020 | Belgium | 345 | 69 ± 14 | 48% | Cohort study | Known or newly diagnosed diabetes (HbA1c ≥ 6.5% on admission) | Mortality | Positive RT-PCR and/or SARS-CoV-2 pneumonia on admission (infiltrates on either chest x-ray or chest-CT) | Single center study, patients with known or newly diagnosed diabetes and confirmed COVID-19 from Mar-May,2020 | 6d,54,55 | HR 0.43 (CI 0.16–1.17) |
| Tchang et al. 2021 | USA | 3,533 | 65(53‐77) * | 59% | Cohort study | EHR/ HbA1c ≥ 6.5%. | Composite outcome: ICU admission, invasive mechanical ventilation, or in‐hospital mortality | Positive RT-PCR | Patients admitted to New York Presbyterian hospital network, between Mar-May 2020 | 1,2,3,6a,6c,6d,6e,19 | HR 1.15 (1.01‐1.30) |
| Wang et al. 2021 | China | 2433 | 60(50–68) * | 50% | Cohort study | EHR | Disease progression and mortality | Positive RT-PCR or serum IgM-IgG antibody detection | Single center study of all COVID-19 positive between Feb-Apr, 2020 | 1,17,18,20,27, 30,32,34,36,37@. 1,32,34a,38@@ | Blood glucose (Ref: 3.9–6.1 mmol/L) Outcome of Disease progression@ > 6.1 mmol/L HR 1.58 (1.25-1.98) < 3.9 mmol/L HR 1.65 (0.97-2.81) Outcome of mortality @@ > 6.1 mmol/L HR 3.22 (1.54-6.73) < 3.9 mmol/L HR 7.31 (0.00, inf) |
| Wang et al. 2020 | China | 605 | 59(47-68) * | 53% | Cohort study | EHR/FBG≥7.0 mmol/L | 28-day mortality and in hospital complications | Positive RT-PCR | Multicenter study, Patients hospitalized with COVID-19 from Jan-Feb 2020 | 1,2,40$ | 28-day mortality$ HR 2.30 [1.49-3.55) 28-day in hospital complications OR 3.99 (2.71, 5.88) (Ref:FBG <6.1 mmol/l) |
| Petrilli et al. 2020 | USA | 2741 | 63(51-74)* | 61% | Cohort study | EHR | critical illness, defined as a composite of care in the ICU, use of mechanical ventilation, discharge to hospice, or death | Positive RT-PCR | Patients tested for SARS-Cov-2 between Mar-Apr 2020 | 1,2,3,4,14, 15,17,19,20, 24,34b,38,44 | OR 1.38 (1.17 to 1.62) (Unadjusted) OR 1.23(0.99-1.5) (Adjusted) |
| Cheng et al. 2021 | China | 407 | 48 | 48% | Cohort study | Fasting glucose ≥ 7.0 mmol/L; 2 hr PP/random glucose ≥ 11.1 mmol/L | ICU admission, Invasive ventilation | Positive RT-PCR in suspected cases with H/O contact with the Wuhan area or with confirmed cases in the last 14 days)/ fever and/or respiratory tract symptoms /radiological features of pneumonia | Multicenter study, patients admitted from Jan-April 2020 | 1 and 23** | ICU admission OR 0.04 (0.00- 0.99)-Preadmission metformin use ** OR 1.02 (0.98–1.05)-Preadmission insulin OR 0.53 (0.18–1.61)-In-hospital metformin OR 1.02 (0.98–1.05)-In-hospital insulin Invasive ventilation Pre-admission metformin-0.09(0.00–1.80) Pre-admission insulin-1.03(1.00–1.07) In-hospital metformin-0.34 (0.07–1.62) In-hospital insulin 1.04 (0.99–1.09) ** |
| Gregory et al. 2021 | USA | 6,451 | T1DM 37(21–51) *T2DM 58(49–97) * | T1DM-42% T2DM-56% | Cohort study | T1DM-H/O autoantibodies or required multiple daily injections. T2DM-on antidiabetic medications | Hospitalization and illness severity | Positive RT-PCR | Epic Clarity data warehouse (houses entire EHR at VUMC, a network of 137 primary care, urgent care, and hospital facilities b/w Mar-Aug 2020. | 1,2,3,6c,15,19 | Hospitalization OR 3.90 (1.75–8.69) - T1DM OR 3.36 (2.49–4.55) -T2DM (Ref- No Diabetes) Illness severity OR 3.35 (1.53–7.33)- T1DM OR 3.42 (2.55–4.58)- T2D (Ref- No Diabetes) |
| Vargas-Vázquez et al, 2020 | Mexico | 317 | 57(47–64) * | NR | Cohort study | DM: previous diagnosis and/or treatment with glucose-lowering agents. Undiagnosed: if HbA1c ≥6.5% De novo/ intrahospital hyperglycemia if FPG ≥140 mg/dL without diabetes and normal HbA1c | Severe COVID-19: composite of death/ICU admission/mechanical ventilation | Positive RT-PCR | Hospitalized patients from a Mexico City reference center | 1,2,15,26 | UndIagnosed T2DM OR: 7.91 (2.59-28.07) Previously diagnosed T2DM OR:3.14 (1.12 10.31) De novo-OR 4.36 (1.53 to 13.67) but not associated with mortality after covariate adjustment |
| Koh et al. 2021 | Singapore | 1,042 | 39±11 | 95% | Cohort study | EHR, HbA1c ≥6.5% | 4 outcomes: a) Severe COVID‐19 [SpO2 ≤93% on room air, respiratory rate ≥30 24 or need for ICU care] b) Dyspnea c) ICU admission. d) length of stay | Positive RT‐PCR throat/nasopharyngeal swab | Single center study with patients admitted from Feb-May 2020 | 1,2,15,17,42 | a) HR: 2.71 (1.34–5.47) b) HR 2.34 (1.13–4.88) c) 6.15 (1.99–19.05) d)1.70 (0.51–2.88) |
| Mithal et al. 2021 | India | 401 | 54(19–92) * | 69% | Cross-sectional study | H/O diabetes or HbA1c ≥ 6.5%. | a) ICU admission b) mortality c) COVID-19 disease severity score (using WHO ordinal scale for clinical improvement) | Positive RT-PCR-nasopharyngeal swab | Single center, patients hospitalized with COVID-19 | None | DM versus no DM a)24.3 vs 12.3%, p-0.002 b)6.3 vs 1.4%, p-0.015 c)20.1 vs 9%, p-0.002. Baseline Hba1c (n = 331) with severity scores (r = 0.136, p = 0.013). |
| Tang et al. 2021 | China | 197 | 66(7–76) * | 61% | Cohort study | Not reported | Disease aggravation | Positive RT-PCR/serum IgM-IgG antibody | Single center, patients hospitalized with COVID-19 from Jan-Mar 2020. | 1,2,6c,11,14, 17,18,20,24, 27,33-39 | 27/88 (30.7%) with disease aggravation were diabetic OR 8.31(2.92–23.6) |
| Zhang et al. 2020 | China | 312 | 57 (38-66) * | 45% | Cohort study | FPG ≥7.0 mmol/L/self-reported physician-diagnosed diabetes/anti-diabetic medication use. Newly diagnosed diabetes: Patients with no H/O diabetes with FPG ≥7.0 mmol/L at hospital admission | a) composite end-point events (including mechanical ventilation, admission to intensive care unit, or death) b)mortality c)mechanical ventilation | Positive RT-PCR/serum IgM-IgG antibody | Multicenter study, Patients hospitalized with COVID-19 from Jan-Mar 2020 | 1,2,12,43-46 | a) HR 2.18 (0.89–5.31) b) HR 6.87 (1.92–24.58) c)2.31 (0.76–7.03) |
| Zheng et al. 2021 | China | 71 | 63±10-DM 54±14-no DM | 64%-DM 38%-no DM | Cohort study | EHR | a) CD4+ T cell% b) CD8+ T cell %. c)IL-6, IL-2, IL-10, and INF-γ d) average hospitalization days | Positive RT-PCR -throat swab/CT findings of COVID-19/SARS-CoV-2 IgM/IgG antibody | Single center study, with COVID positive inpatients from Feb-Mar 2020 | 15 | a)51.75 ± 4.45‡§ b)20.95 ± 7.61‡ c) upregulation† d)13 (9, 17.50) ‡§ |
ARDS, Acute respiratory distress syndrome;
Adjustments: 1=Age; 2=Sex; 3=Race; 4=Ethnicity; 5=Obesity; 6=Comorbidities; 6a=chronic respiratory disease 6b=Asthma; 6c=HT; 6d=chronic heart disease/cardiovascular disease/CAD; 6e=chronic renal disease;
6f=chronic liver disease; 6g=chronic neurological disease; 6h=Immunosuppression; 6i=COPD; 6j=heart failure; 6k=Atrial fibrillation; 6l=hematological disorders; 6m=rheumatologic/autoimmune disorder;
7=deprivation; 8=geographical region; 9=previous hospital admissions with coronary heart disease, cerebrovascular disease, or heart failure; 10=History of diabetes; 11=LFT; 12=eGFR; 13=glucose on admission;
14=NT‐proBNP (per 100 pg/mL); 15=BMI; 16=History of cancer;17=CRP; 18=LDH (per 100 U/L); 19=smoking; 20=Lymphocyte count (per 1 × 109/L); 21=WBC count; 22=Treatment with ACE/ARB's; 23=blood glucose;
24=Serum ferritin (per 100 μg/L); 25=HbA1c; 26=Number of comorbidities; 27=Differential neutrophil count; 28=Hematuria; 29=Initial serum globulin (g/L); 30=Fever with chills; 31=Marijuana use;
32=Platelet count (x109 cells/L); 33=CD3, CD4, CD8; 34=Coagulation function (34a, Fbg; 34b, D dimer); 35=IL-6; 36=Chest distress/dyspnea/chest tightness; 37=BUN; 38=Creatinine kinase; 39=CTnI;
40=CRB-65 measures the severity of pneumonia on a 0 to 4 scale; 41=QTc prolongation; 42=≥2 comorbidities; 43=Prothrombin time; 44=Procalcitonin; 45=aspartate aminotransferase (AST);
46=hospital; 47=systolic blood pressure; 48=total cholesterol; 49=antihypertensive drugs, lipid-lowering agents; 50=admission to ICU; 51=invasive mechanical ventilation;
52=glucose-lowering medication before and during hospital admission; 53=corticosteroid use; 54=cognitive impairment;55=Area of lung injury>50%
Median (IQR);
composite of risk factors including diabetes was the exposure; % adjustment with each outcome based on P < 0.05
versus no DM P < 0.05
versus IFG P < 0.05;
Diabetes Status and Risk of Poor Outcomes or Mortality
Most published studies to date, included only hospitalized patients with confirmed SARS-CoV-2 infection. Table II provides a summary of all studies and their key design features and findings. In total 26 inpatient studies were identified among which 20 reported mortality outcomes while the remaining 6 reported adverse outcomes such as ICU admission and/or length of stay, need for mechanical ventilation, and/or COVID-19 severity. Reports arose from 11 countries across diverse populations and included 28,311 patients. Reported mortality rates varied greatly but among eight such studies with a samples size of at least 1000, six studies reported mortality rates >20%. Among 13 studies that reported a summary measure of association for mortality, 9 observed a statistically significantly increased risk for death among those with vs. without diabetes. Limited reporting for T1 vs. T2 diabetes was available, although at least one large study with >6000 patients21 reported empirically higher risk for hospitalization among patients with T1DM (but seemingly equal risk for mortality between T1DM and T2DM). In contrast, a separate study with only 238 patients reported lower mortality risk among T1DM although there was only 1 death in the T1DM group and the findings for both T1DM and T2DM were not statistically significant.
Two studies with sample sizes sufficient for subgroup analyses observed that the mortality risk among people with vs. without diabetes, decreased as age increased. Woolcott et al19 reported adjusted hazard ratios for death to 3.12 (2.86-3.40) for patients 20-39 years of age compared to 1.11 (95% CI 1.06-1.16) for patients 80 years of age or older (P-value for interaction<0.05). Similarly, Dennis et al22 observed adjusted hazard ratios for mortality among patients with vs. without diabetes to be 1.50(1.05-2.15), 1.29(1.10-1.51), and 1.18(1.09-1.29) among patients aged 18-49, 50-64, and 65+ respectively (P-value for interaction<0.05). These interactions could be biologically driven although the underlying mechanisms are unclear. Alternatively, these findings are quite possibly a consequence of scaling as the measures of association were relative risk measures and not absolute risk differences. Regardless, despite attenuated relative risk in the oldest age groups, risk remained elevated among patients with vs. without diabetes and absolute risk for poor outcomes in older, SARS-CoV-2 infected patients with diabetes is quite high.
Impaired glucose regulation and SARS-CoV-2
There were nine studies identified that assessed impaired glucose regulation and SARS-CoV-2 infection (Table III). The criteria used to define impaired glucose regulation varied considerably making between study comparisons challenging. Some studies used objective laboratory parameters while others relied on self-report physician diagnosis. Even among studies relying on laboratory measures, there was variation in the analyses (eg, fasting blood glucose, HbA1c, and triglyceride and glucose index) and thresholds defining IGR. Most studies reported outcomes to be worse among patients with non-diabetic IGR vs metabolically normal patients. However, study samples were small with seven of nine studies enrolling fewer than 500 patients and statistical significance was lacking in several reports.
Table III.
Summary of studies assessing the association between non-diabetic impaired glucose regulation and SARS-CoV-2 infection and/or COVID-19 outcomes
| Study | Country | N | Mean Age±SD (range) | Male (%) | Study design | Prediabetes/IFG assessment | Outcome | SARS-CoV-2 Assessment | Study population | Adjustments | Measure of Association (95% Confidence Interval) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mamtani et al. 2021 | USA | 403 | 55 | 68% | Cohort study | Hyperglycemia [atleast one blood glucose ≥7.78 mmol/L during hospitalization with no history of diabetes | Mortality | Positive PCR for the RdRp and N genes. | Single center study, COVID-19 patients hospitalized from March-May 2020 | 1,27-32 | OR 21.94 (4.04–119.0) |
| Wanget al. 2020 | China | 605 | 59 (47-68)* | 53% | Cohort study | EHR/FBG 6.1-6.9 mmol/l | 28-day mortality and in hospital complications | Laboratory-confirmed in accordance with the interim guidance formulated by the WHO | Multicenter study, Patients hospitalized with COVID-19 from Jan-Feb 2020 | 1,2,40$ | 28-day in hospital complications OR: 2.61 (1.64, 4.41) 28-day mortality$ HR 1.71 (0.99, 2.94) Ref:FBG <6.1 mmol/l |
| Zhang et al. 2020 | China | 312 | 57 (38-66)* | 45% | Cohort study | IFG - glucose b/w 5.6 -6.9 mmol/L. | a) composite end-point events (including mechanical ventilation, admission to ICU/death) b)mortality c)mechanical ventilation | Positive RT-PCR of nasopharyngeal swab or positive serum specific IgM and IgG antibody |
Multicenter study, Patients hospitalized with COVID-19 from Jan-Mar 2020 | 1,2,11,12, 43-46 |
a) 1.21 (0.43–3.39) b) HR 4.06 (1.00–16.42) c)0.95 (0.25–3.66) |
| Sourij et al. 2021 | Austria | 238 | 71±13 | 64% | Cohort study | HbA1c 39-46 mmol/mol | Mortality | Positive throat swab for SARS‐CoV‐2 | Hospitalized, with COVID-19, and either diabetes/ prediabetes | None | Prediabetes: 14.9% versus Diabetes:26.7% (p=0.128) |
| Li et al. 2020 | China | 453 | 61 (49-68)* | 53% | Cohort study | Hyperglycemia (fasting glucose 5.6-6.9 mmol/L and/or HbA1c 5.7-6.4%) | Mortality | Exposure to confirmed SARS-CoV-2 infection or to the Wuhan Huanan seafood market. |
Patients admitted with lab‐confirmed SARS‐Cov‐2 infection from Jan-Mar 2020. | 1,2,19,47-53 | HR: 2.64 (0.50-14) |
| Ren et al. 2020 | China | 151 | 59±16 | 52% | Cohort study | EHR-Triglyceride and glucose index (TyG)-marker for insulin resistance | Severe covid 19 infection and mortality | Severe: 1. respiratory rate > 30/min, 2. oxygen saturation ≤ 93%, 3. PaO2/FiO2, 4. Patients developed either with shock, or respiratory failure requiring mechanical ventilation, or combined with the other organ failure admission to ICU |
Hospitalized with COVID-19, from Jan-Feb,2020 | 1,2,17,26, 31 | OR 2.9 (1.2-6.3)- severity OR 2.9 (1.2-6.7)-for mortality |
| Vargas-Vázquez et al. 2020 | Mexico | 317 | 57 (47–64)* | NR | Cohort study | using HbA1c ADA criteria | Severe COVID-19: composite of death, ICU admission or mechanical ventilation | Positive RT-PCR | Hospitalized patients from a Mexico City reference center | 1,2,15,26 | OR 3.25 (1.20-10.46) |
| Koh et al. 2021 | Singapore | 1,042 | 39±11 | 95% | Cohort study | EHR, HbA1c 5.7–6.4% | Severe COVID-19 | Positive RT‐PCR throat/nasopharyngeal swab | Single center study with patients admitted from Feb-May,2020 | 1,2,15,17, 42 | HR: 0.49 (0.11–2.24) |
| Zheng et al. 2021 | China | 71 | 61±14(IFG) 54±14(no DM) | 44% (IFG) 38% (no DM) | Cohort study | EHR | Hospitalization days, mortality | Positive RT-PCR -throat swab or typical CT findings of COVID-19 or SARS-CoV-2 IgM/IgG antibody | Single center study, with COVID positive inpatients from Feb-Mar 2020 | 15 | IFG no different than normal for hospitalization days, or mortality. |
Adjustments: 1=Age; 2=Sex; 3=Race; 4=Ethnicity; 5=Obesity; 6=Comorbidities; 6a=chronic respiratory disease 6b=Asthma; 6c=HT; 6d=chronic heart disease/cardiovascular disease/CAD; 6e=chronic renal disease;
6f=chronic liver disease; 6g=chronic neurological disease; 6h=Immunosuppression; 6i=COPD; 6j=heart failure; 6k=Atrial fibrillation; 6l=hematological disorders; 6m=rheumatologic/autoimmune disorder;
7=deprivation; 8=geographical region; 9=previous hospital admissions with coronary heart disease, cerebrovascular disease, or heart failure; 10=History of diabetes; 11=LFT; 12=eGFR; 13=glucose on admission;
14=NT‐proBNP (per 100 pg/mL); 15=BMI; 16=History of cancer;17=CRP; 18=LDH (per 100 U/L); 19=smoking; 20=Lymphocyte count (per 1 × 109/L); 21=WBC count; 22=Treatment with ACE/ARB's; 23=blood glucose;
24=Serum ferritin (per 100 μg/L); 25=Hba1c; 26=Number of comorbidities; 27=Differential neutrophil count; 28=Hematuria; 29=Initial serum globulin (g/L); 30=Fever with chills; 31=Marijuana use;
32=Platelet count (x109 cells/L); 33=CD3, CD4, CD8; 34=Coagulation function (34a,Fbg; 34b,D dimer); 35=IL-6; 36=Chest distress/dyspnea/chest tightness; 37=BUN; 38=Creatinine kinase; 39=CTnI;
40=CRB-65 measures the severity of pneumonia on a 0 to 4 scale; 41=QTc prolongation; 42=≥2 comorbidities; 43=Prothrombin time; 44=Procalcitonin; 45=aspartate aminotransferase (AST);
46=hospital; 47=systolic blood pressure; 48=total cholesterol; 49=antihypertensive drugs, lipid-lowering agents; 50=admission to ICU; 51=invasive mechanical ventilation;
52=glucose-lowering medication before and during hospital admission ; 53=corticosteroid use; 54=cognitive impairment;55=Area of lung injury>50%
Median (IQR).
METHODOLOGICAL LIMITATIONS OF EXISTING STUDIES
Selection Bias
Many of the studies in this review included only hospitalized patients. Such studies are potentially susceptible to differential selection bias according to diabetes status. In some settings, it is possible that – all else equal – patients with diabetes are potentially more likely to be tested for SARS-CoV-2 and/or admitted as the result of perceived higher risk. This could result in a bias such that the risk profile of admitted DM patients was more favorable compared to non-DM patients who perhaps required more severe infection for admission. Such bias could attenuate study findings. While selection bias is a separate issue from confounding, it is possible to mitigate these biases analytically through statistical adjustment if sufficient data collection is available23 particularly on factors related to prognosis. Given the limited adjustment in most studies, selection bias is a concern that remains to be addressed in future studies.
Information Bias Vis-à-vis SARS-CoV-2 Case Definitions
The assessment method for SARS-CoV-2 infection varied across studies. Most studies defined infection as physician diagnosed with a positive nasopharyngeal and/or oropharyngeal swab RT-PCR confirmed infection. However, several studies (particularly those from early in the pandemic when testing capacity was limited) often relied on physician diagnosis based on symptomology and radiological findings suggestive of SARS-CoV-2 infection. Information bias of this nature is likely to bias study findings towards the null as the SARS-CoV-2 positivity rates during most of the pandemic has been <50% and frequently <5% among symptomatic individuals.24
No studies were identified that considered asymptomatic SARS-CoV-2 infection. The lack of population-based research that systematically monitors for any infection (asymptomatic or otherwise) is a major limitation in the literature to date. In the context of risk factor studies, such as those considering metabolic parameters as a risk factor for SARS-CoV-2 infection, mildly symptomatic and asymptomatic infections can bias study results by misclassifying participants as uninfected when in fact they were truly infected but not diagnosed. This form of classical information bias in epidemiological studies will generally bias towards the null if it occurs equally among those with vs. without the risk factor of interest (eg, diabetes).
It remains poorly understood whether asymptomatic cases are more or less likely to occur among individuals with IGR. This is in part due to the need for costly serial testing to enhance the likelihood of detecting asymptomatic and/or mildly symptomatic cases, since gold-standard PCR testing can only detect active viral infection. To address this issue, an alternative to frequent (∼weekly) PCR testing, is less frequent antibody (∼bi-monthly) testing to assess recent history of infection. While promising, important limitations to this approach also remain. For example, the lack of definitive knowledge about the duration of antibody responses coupled with the large number of assays available with substantial performance variability is an important current limitation.25 Additionally, lack of data related to antibody response and duration that might be differential by IGR status is another important concern. Ironically, while antibody studies might be conceptually helpful for identifying prior asymptomatic infections, if their performance is dependent on IGR status, their use would simply lead to another form of differential misclassification bias. Published data on this topic are conflicting with at least one study suggesting antibody response to be similar26 among those with vs. without diabetes while another study reported impaired seroconversion27 in diabetes (which would lead to SARS-CoV-2 under-estimation in diabetes). Finally, in the age of vaccination, assays that can reliably distinguish natural infection from vaccination based on serology, will be necessary.28 Ultimately, while reliance on antibody assays alone could be problematic, their use in combination with other assessment methods could enhance sensitivity of infection identification methods.
CONFOUNDING
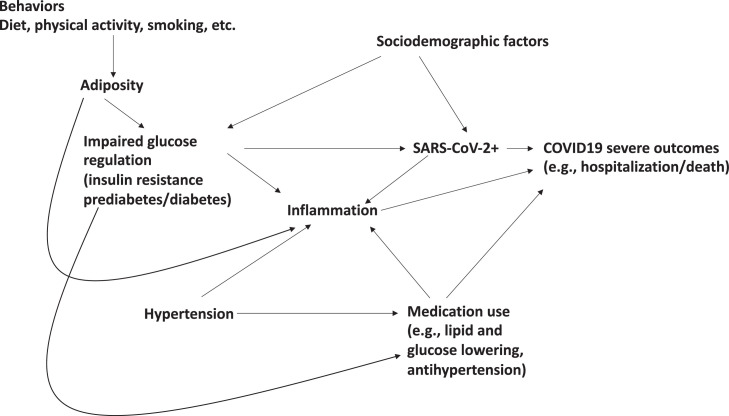
All studies included in this review were observational study designs which are susceptible to confounding, in which associations between IGR status and either SARS-CoV-2 infection, or poor COVID-19 outcomes, might be spurious and due to differential distributions of causal risk factors for outcomes among those with vs. without IGR. The epidemiological study design that is least susceptible to confounding is a randomized controlled trial. However, for hypotheses of IGR status as a risk factor for infection and infection severity, RCTs are not possible as the IGR status in humans cannot be randomized. Therefore, well conducted observational studies will be necessary to inform causality. Of central importance to addressing this limitation is the robust data collection of including important potential confounders. The relevant data collection likely extends beyond standard data available in medical records and includes information on lifestyle. As importantly, many of these factors should be assessed prior to infection (and prior to pandemic times) to ensure that baseline risk is rigorously characterized (Fig 2. ).
Fig 2.
Directed Acyclic Graph summarizing possible causal pathways linking behaviors, biomarkers, IGR, comorbidities to SARS-CoV-2 infection and severity.
Overall, the level of confounder adjustment observed in most reported studies (Tables II and III) was quite limited. For example, only one-third of studies adjusted for either adiposity or smoking and fewer adjusted for both. Lack of adiposity adjustments is particularly concerning given the early data that adiposity is likely a strong risk factor for diabetes, as well as for hospitalization and death 29 among patients diagnosed with COVID-19. (Fig 2) Similarly, rigorous adjustment for medication use was also limited. Xian et al demonstrated in mouse models, the anti-inflammatory role of metformin via inhibition of the NLRP3 inflammasome and interlekin-1β induced acute respiratory distress syndrome in SARS-CoV-2 infection.30 Similarly statins such as simvastatin and lovastatin were found to be most effective in severe SARS-CoV-2 infection exerting its influence via cytotoxic T lymphocytes and regulatory T cell induction.31 As the literature assessing the effect of medications targeting dysglycemia, hypertension, thrombosis,32 and hypercholesterolemia on SARS-CoV-2 infection and severity tends to suggest modest protection against infection and mortality, future studies will be enhanced by accounting for medications to avoid negative confounding towards the null (eg, weaker associations due to the protective effect of medications being more common among patients with vs. without diabetes). The need for more robust consideration of several additional important factors including socio-demographic (access to care, income, education, isolation), behavioral (diet, activity), (Fig 2) and biological (d-dimer, blood pressure, renal function) was also largely lacking in published studies limiting interpretation and conclusions.
OVERVIEW OF BIOLOGICAL PLAUSIBILITY LINKING SARS-COV-2 AND METABOLIC ABNORMALITIES
Impaired glucose regulation contributing to SARS-CoV-2 infection
The systematic review discussed above supports the preliminary mechanistic evidence linking IGR to risk for infection as briefly discussed below.
First, SARS-CoV-2 is postulated to bind to ACE-2 (Angiotensin Converting Enzyme-2) receptor which is found on human lung epithelium, vascular endothelium, intestinal lining, pancreatic beta cells etc.12 ACE-2 works in conjunction with the cell surface protein, Transmembrane serine protease 2 (TMPRSS2), facilitating cellular entry of SARS-CoV-2,33 resulting in an increased viral load in patients with impaired glucose regulation. Once the virus enters, the S protein activates Desintegrin and metalloproteinase domain 17 (ADAM 17) which has been postulated to increase insulin resistance34 as well as downregulate surface ACE-2 receptor expression. The current literature on receptor expression on the pancreatic cells is still under speculation. For instance, Lau et al demonstrated via RT-PCR and western blot in mouse pancreatic cells, the distribution of ACE, angiotensin 1 (AT-1) and angiotensin 2 (AT-2). The authors concluded that ACE, AT-1, AT-2, and angiotensinogen were expressed in endogenous islets while AT-1 was localized to pancreatic beta cells.35 On the other hand, Liu et al, illustrated that ACE-2 was expressed both in exocrine gland and islet cells. In support of these findings, pancreatic injury in the form of elevated amylase and lipase levels, along with radiological evidence of localized enlargement of the pancreas as well as pancreatic duct dilatation was observed in patients infected with SARS-CoV-2.36 Another study looked at ACE-2 and TMPRSS2 expression in the islets of Langerhans which was observed via fluorescence stain of five histologically healthy human pancreas. The expression of ACE-2 varied among different cells of the pancreas. For example, TMPRSS2 was detected mainly in endocrine cells, to a lesser extent in the ductal cells and uncommon in the acinar cells. Overall, this study demonstrated insulin secretory granules in beta cells were impaired in those infected with SARS-CoV-2 along with impaired glucose mediated insulin secretion. They were able to demonstrate that the infected islet cells stained positive for endocrine markers (PDX1, NKX6.1) but were negative for insulin indicative of susceptibility of the islet's cells to SARS-CoV-2 infection.37 However, conflicting evidence exists with one study observing that ACE-2 is mainly expressed on exocrine cells and islets of the pancreas while TMPRSS2 is expressed on ductal cells, diminishing the earlier hypothesis of co-expression of both receptors on islet cells.38 Taneera et al demonstrated that although pancreatic islets expressed ACE-2 and TMPRSS2 irrespective of diabetes status, ACE-2 expression was increased in diabetes, while upregulation of TMPRSS2 was only observed in obese individuals and those with elevated Hba1c levels alone.39 Future studies are needed to fully understand the synergistic role of these receptors resulting in disruption of glucose homeostasis for identifying risk factors as well as effective management of SARS-CoV-2 infection.
Secondly, diabetes has shown to significantly alter cell-mediated immunity40 , 41 lowering the chemotactic and phagocytic function leading to a diminished response to infection. For example, impaired interferon production (IFN-alpha and IFN-gamma) was observed in non-obese diabetic and prediabetic mouse models.42 Another hypothesized mechanism is that elevated glucose levels increase adherence of microorganisms to cellular receptors,43 thereby increasing the prevalence of infections in patients with diabetes. Increased SARS-CoV-2 viral replication and cytokine expression were also observed in monocytes of diabetic and/or obese patients in an environment of elevated glucose levels.44 Codo et al observed that the hypoxia-inducible factor 1 alpha(HIF1A) was overexpressed in the monocytes of SARS-CoV-2 infection in greater intensity that that of RSV or influenza-infected monocytes.44 This might explain the severity of COVID-19 infection and poorer outcomes commonly reported in patients with diabetes.45 The concurrent responses resemble that of the severe and prolonged infective period observed in MERS-CoV infected mice models with diabetes.46
Despite, a reasonable biological premise for IGR to increase risk for poor outcomes among SARS-CoV-2 infected individuals, future studies are necessary to: (1) confirm biological mechanisms in animal models and extend findings to humans; (2) identify biomarker phenotypes that identify elevated risk; and (3) translate these findings to interventional approaches that reduce infection risk and improve outcomes post-infection.
SARS-CoV-2 infection as a contributor to impaired glucose regulation
There are also several biologically plausible mechanisms through which SARS-CoV-2 might predispose to the development of impaired glucose regulation (as opposed to the reverse). Key mechanisms discussed to date are outlined below.
Chronic inflammation is a plausible biological mechanism linking infections and insulin resistance. Animal models have shown that inflammatory cytokines such as TNF-α can induce a state of insulin resistance,47 possibly as a consequence of TNF-α’s ability to interrupt serine phosphorylation of IRS-1,48 and data in humans have repeatedly shown inflammation to be an independent risk factor for both insulin resistance49 and T2DM.50, 51, 52 If a chronic inflammatory phenotype emerges in some COVID-19 patients, it is plausible that insulin resistance may follow along with increased risk for incident prediabetes of diabetes. Notably, in studies that lack pre-infection phenotyping, it is not possible rule out reverse causality (i.e., SARS-CoV-2 induced IGR rather than IGR increase risk for infection and poor outcomes).
SARS-CoV-2 induction of endothelial dysfunction is another potential mechanism underlying poor metabolic outcomes among individuals with symptomatic COVID-19. Evidence is accumulating suggesting that SARS-CoV-2 causes endothelial dysfunction.53 Importantly, endothelial dysfunction is believed to directly contribute to insulin resistance, possibly by impairing transcapillary passage of insulin to target tissues.54 This primarily occurs through viral infection induced activation of the integrated stress response pathway (ISR). Phosphorylation of the IRS1 receptor by IRS regulator, PKR kinases, can attenuate insulin action, leading to insulin resistance.55
Tropisms for beta-cells is another mechanism linking infection to metabolic abnormalities.37 , 56 , 57 Very recent evidence suggests that SARS-Cov-2 can infect human beta-cells leading to morphological, transcriptional, and functional changes such as reduced insulin-secretory granules and reduced glucose stimulated insulin secretion.37 , 56 Accordingly, numerous prior studies have hypothesized a variety of viral infections in the etiology of T1DM due to beta-cell infection and impairment.58 In support of this notion, impaired glucose metabolism and acute-onset diabetes have been reported to be higher among those with SARS coronavirus 1 pneumonia as compared to individuals with non-SARS pneumonia.59
Most of the aforementioned suggested mechanisms linking infection to incident IGR are based on conceptual models, animal models or are extrapolated from other infection models. Although much of the data available stems from animal models, there is insufficient evidence demonstrating SARS-CoV-2 infection causes abnormal glucose levels and findings to date are limited to involvement of the respiratory system and transient systemic inflammation.60 Moreover, limited human data are available explicitly testing whether SARS-CoV-2 infection increases risk for incident IGR in the short or long-term. As research on Post-Acute Sequelae of SARS-CoV-2 infection (PASC) emerges, studies informing metabolic outcomes will be important for identifying at risk populations requiring careful monitoring and screening.
CONCLUSION
The current literature suggests that a bidirectional relationship between impaired glucose regulation and SARS-CoV-2 infection might exist.
A total of 32 human studies were identified on the topic of IGR as predictor of infection or poor outcomes among infected patients. The majority of studies identified, support the hypothesis that IGR increases risk for poor outcomes among patients with COVID-19. However, few studies addressed risk for infection and even fewer human studies considered SARS-CoV-2 infection as a risk factor for the development of new onset IGR. Additionally, only a limited number of studies considered prediabetes and insulin resistance as possible risk factors for infection. This is an important area of research as SARS-CoV-2 becomes endemic in much of the world for the foreseeable future. In the U.S. context, nearly one in three US adults have prediabetes61 but limited guidance on infection control measures are available to individuals with prediabetes. As factors such as prediabetes and insulin resistance are modifiable, they are potential targets for reducing the burden of SARS-CoV-2 and setting vaccination policy vis-à-vis patient priority. Additionally, future studies need to account for the use of medications commonly used among patients with prediabetes or diabetes to obtain clear estimates of risk.
Although most research to date supports bidirectional relationships between impaired glucose regulation and SARS-CoV-2 infection and outcomes, several of the aforementioned limitations preclude strong conclusions and future studies are necessary to more rigorously form causal conclusions and prevention-oriented policy. Doing so will not only benefit the clinical management of patients with SARS-CoV-2 but also the broader public health effort to reduce the burden of infection on population health.
ACKNOWLEDGMENTS
Conflict of Interest: All authors have read the journal's policy on disclosure of potential conflicts of interest. The authors have declared that no conflict of interest exists. S.R., R.D. conceptualized, wrote, and edited the manuscript. All authors have read the journal's authorship agreement and that the manuscript has been reviewed by and approved by all named authors.
References
- 1.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 3.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 5.Siu AL, Force USPST. Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:861–868. doi: 10.7326/M15-2345. [DOI] [PubMed] [Google Scholar]
- 6.Mechanick JI, Garber AJ, Grunberger G, Handelsman Y, Garvey WT. Dysglycemia-Based Chronic Disease: An American Association of Clinical Endocrinologists Position Statement. Endocr Pract. 2018;24:995–1011. doi: 10.4158/PS-2018-0139. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research G The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371:741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demmer RT, Breskin A, Rosenbaum M, et al. The subgingival microbiome, systemic inflammation and insulin resistance: The Oral Infections, Glucose Intolerance and Insulin Resistance Study. J Clin Periodontol. 2017;44:255–265. doi: 10.1111/jcpe.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Real JM, Lopez-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- 11.Yki-Jarvinen H, Sammalkorpi K, Koivisto VA, Nikkila EA. Severity, duration, and mechanisms of insulin resistance during acute infections. J Clin Endocrinol Metab. 1989;69:317–323. doi: 10.1210/jcem-69-2-317. [DOI] [PubMed] [Google Scholar]
- 12.Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the study of health in Pomerania (SHIP) Diabetes Care. 2010;33:1037–1043. doi: 10.2337/dc09-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecube A, Hernandez C, Genesca J, Simo R. Glucose abnormalities in patients with hepatitis C virus infection: Epidemiology and pathogenesis. Diabetes Care. 2006;29:1140–1149. doi: 10.2337/diacare.2951140. [DOI] [PubMed] [Google Scholar]
- 14.Jeon CY, Haan MN, Cheng C, et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520–525. doi: 10.2337/dc11-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demmer RT, Jacobs DR, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–1379. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlievert PM, Salgado-Pabon W, Klingelhutz AJ. Does Staphylococcus aureus have a role in the development of Type 2 diabetes mellitus? Future Microbiol. 2015;10:1549–1552. doi: 10.2217/fmb.15.95. [DOI] [PubMed] [Google Scholar]
- 17.McGurnaghan SJ, Weir A, Bishop J, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolcott OO, Castilla-Bancayan JP. The effect of age on the association between diabetes and mortality in adult patients with COVID-19 in Mexico. Sci Rep. 2021;11:8386. doi: 10.1038/s41598-021-88014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverii GA, Monami M, Cernigliaro A, et al. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metab Cardiovasc Dis. 2021;31:396–398. doi: 10.1016/j.numecd.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic's Impact in Type 1 and Type 2 Diabetes. Diabetes Care. 2021;44:526–532. doi: 10.2337/dc20-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis JM, Mateen BA, Sonabend R, et al. Type 2 Diabetes and COVID-19-Related Mortality in the Critical Care Setting: A National Cohort Study in England, March-July 2020. Diabetes Care. 2021;44:50–57. doi: 10.2337/dc20-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 24.Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11:4507. doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11:3436. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lampasona V, Secchi M, Scavini M, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63:2548–2558. doi: 10.1007/s00125-020-05284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal R, Sachdeva N, Mukherjee S, et al. Impaired anti-SARS-CoV-2 antibody response in non-severe COVID-19 patients with diabetes mellitus: A preliminary report. Diabetes Metab Syndr. 2021;15:193–196. doi: 10.1016/j.dsx.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demmer RT, Baumgartner B, Wiggen TD, et al. Identification of natural SARS-CoV-2 infection in seroprevalence studies among vaccinated populations. medRxiv. 2021 doi: 10.1016/j.mayocp.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xian H, Liu Y, Rundberg Nilsson A, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54:1463–77e11. doi: 10.1016/j.immuni.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkoshi Z. The Binary Model of Chronic Diseases Applied to COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.716084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–80e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiorentino L, Vivanti A, Cavalera M, et al. Increased tumor necrosis factor alpha-converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology. 2010;51:103–110. doi: 10.1002/hep.23250. [DOI] [PubMed] [Google Scholar]
- 35.Lau T, Carlsson PO, Leung PS. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004;47:240–248. doi: 10.1007/s00125-003-1295-1. [DOI] [PubMed] [Google Scholar]
- 36.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020;18:2128–30e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller JA, Gross R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 38.Coate KC, Cha J, Shrestha S, et al. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in beta Cells. Cell Metab. 2020;32:1028–40e4. doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taneera J, El-Huneidi W, Hamad M, Mohammed AK, Elaraby E, Hachim MY. Expression Profile of SARS-CoV-2 Host Receptors in Human Pancreatic Islets Revealed Upregulation of ACE2 in Diabetic Donors. Biology (Basel) 2020;9 doi: 10.3390/biology9080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandraki KI, Piperi C, Ziakas PD, et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol. 2008;28:314–321. doi: 10.1007/s10875-007-9164-1. [DOI] [PubMed] [Google Scholar]
- 41.Pavlou S, Lindsay J, Ingram R, Xu H, Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19:24. doi: 10.1186/s12865-018-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009;126:268–279. doi: 10.1111/j.1365-2567.2008.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darwazeh AM, Lamey PJ, Samaranayake LP, et al. The relationship between colonisation, secretor status and in-vitro adhesion of Candida albicans to buccal epithelial cells from diabetics. J Med Microbiol. 1990;33:43–49. doi: 10.1099/00222615-33-1-43. [DOI] [PubMed] [Google Scholar]
- 44.Codo AC, Davanzo GG, Monteiro LB, et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1alpha/Glycolysis-Dependent Axis. Cell Metab. 2020;32:437–46e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koh H, Moh AMC, Yeoh E, et al. Diabetes predicts severity of COVID-19 infection in a retrospective cohort: A mediatory role of the inflammatory biomarker C-reactive protein. J Med Virol. 2021;93:3023–3032. doi: 10.1002/jmv.26837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20):e131774. doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ling PR, Bistrian BR, Mendez B, Istfan NW. Effects of systemic infusions of endotoxin, tumor necrosis factor, and interleukin-1 on glucose metabolism in the rat: relationship to endogenous glucose production and peripheral tissue glucose uptake. Metabolism. 1994;43:279–284. doi: 10.1016/0026-0495(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 48.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 49.Park K, Steffes M, Lee DH, Himes JH, Jacobs DR. Association of inflammation with worsening HOMA-insulin resistance. Diabetologia. 2009;52:2337–2344. doi: 10.1007/s00125-009-1486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pradhan AD, Cook NR, Buring JE, Manson JE. Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23:650–655. doi: 10.1161/01.ATV.0000065636.15310.9C. [DOI] [PubMed] [Google Scholar]
- 51.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 52.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 53.Jin Y, Ji W, Yang H, Chen S, Zhang W, Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22:423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 55.Santos A, Magro DO, Evangelista-Poderoso R, Saad MJA. Diabetes, obesity, and insulin resistance in COVID-19: molecular interrelationship and therapeutic implications. Diabetol Metab Syndr. 2021;13:23. doi: 10.1186/s13098-021-00639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu CT, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Cell Metab. 2021;33:1565–76 e5. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Lancet Diabetes E COVID-19 and diabetes: a co-conspiracy? Lancet Diabetes Endocrinol. 2020;8:801. doi: 10.1016/S2213-8587(20)30315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14:2211–2217. doi: 10.1016/j.dsx.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehaideb SN, Abdullah ML, Abuyassin B, Bouchama A. Evidence of a wide gap between COVID-19 in humans and animal models: a systematic review. Crit Care. 2020;24:594. doi: 10.1186/s13054-020-03304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999-2010. Diabetes Care. 2013;36:2286–2293. doi: 10.2337/dc12-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]