We report two cases of severe adult-onset Still's disease (AOSD) that presented shortly after receiving the BNT162b2 mRNA Covid-19 Vaccine (Pfizer).
1. Case 1
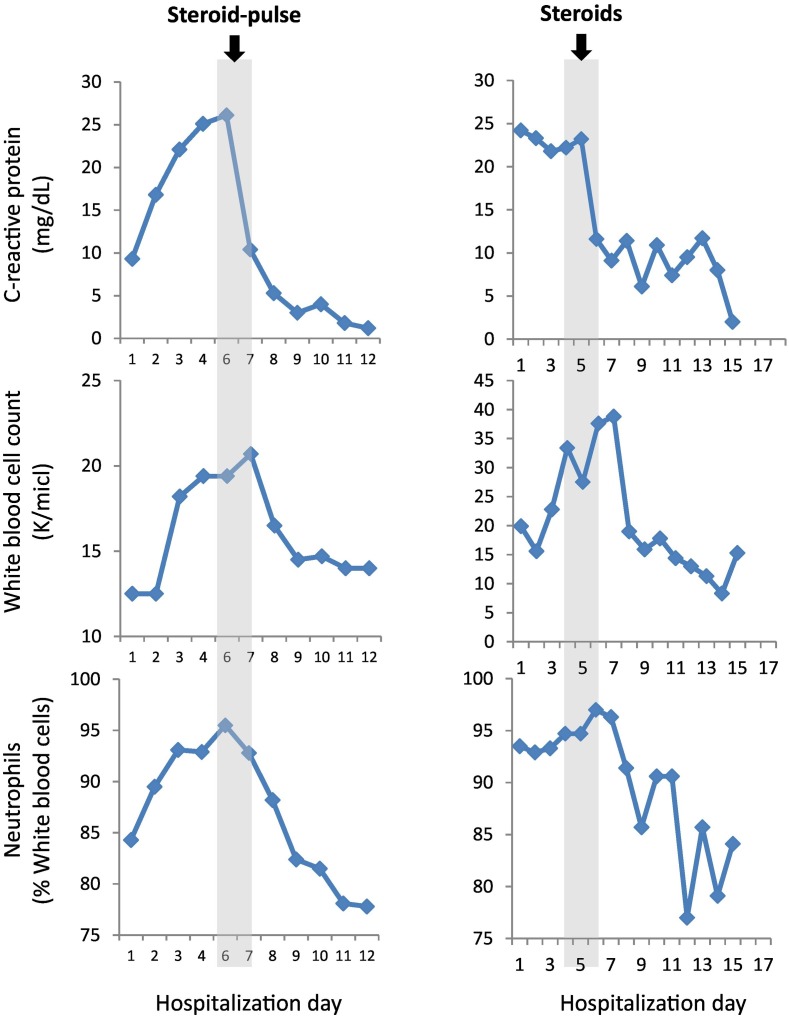
A 43-year-old healthy man complained of headache, malaise, sore throat, and a high-grade fever (39-40 °C) started ten days after receiving the second dose of the BNT162b2 mRNA Covid-19 Vaccine (Pfizer) [1], Pfizer's COVID-19 vaccine. He first was thought to have sinusitis and treated with amoxicillin-clavulanic acid. The patient continued to complain of persistent daily spikes of fever of up to 40 °C and muscle pain despite consistent use of anti-pyretic medications (e.g., acetaminophen, NSAIDs). Later he developed arthralgia of both knees with a large right knee effusion. On admission, the pertinent findings on examination were temperature of 39-40 °C, sinus tachycardia (115 bpm), and large right knee effusion with no evidence of rash. Initial laboratory tests displayed elevated serum C-reactive protein (CRP) of 9.320 mg/dL (reference range 0.000-0.500 mg/dL), leukocytosis of 12.5 K/μL (reference range 4.5–11 K/μL), and a negative COVID-19 nasopharyngeal PCR test. The chest x-ray was unremarkable, and ECG was significant for sinus tachycardia with no ST-T changes. His right knee was punctured and yielded 70 ml yellowish and lucid fluid, which had 3,546 leukocytes, 71% PMNs, with no evidence of crystals on microscopy, as well as negative cultures. He was initially treated with broad-spectrum antibiotics that covered atypical infections. Four days later he continued having high-grade fever and complained of dyspnea and found to be hypoxic requiring a 100% non-rebreather mask. Laboratory blood tests showed further increase in CRP, hypoalbuminemia (decrease from 4.2 to 2.4 g/dL), and mild and transient troponin elevation (up to 52 ng/L then decreased below 13 ng/L, normal ranges 0 - 14 ng/L). Imaging studies were notable for bilateral pleural effusion and diffuse bilateral infiltrates, and no evidence of pulmonary emboli on chest computed tomographic angiography (CTA). ECG showed sinus tachycardia with no ischemic changes. trans-Thoracic echocardiography (TTE) revealed regional wall motion abnormality with mild left ventricular dysfunction (LVEF = 50%), with normal coronary arteries on cardiac CTA (CCTA). High-grade fever persisted and was accompanied with a reddish macular rash on both hips and abdomen margins, and with further elevation of CRP and neutrophilic (>95%) leukocytosis (Fig. 1 ), increased LDH with normal liver enzymes levels. Serology tests were negative for infectious agents (e.g., EBV, CMV, HIV, HBC, HCV, syphilis, Q fever, Brucella, Legionella) as well as autoantibodies (e.g., RF, ANA, and entire ENAs, anti-PR3, anti-MPO). Based on the combination of spiky fever, arthralgia/arthritis, macular rash, leukocytosis with elevated CRP, the patient was diagnosed with AOSD in accordance with the Yamaguchi classification criteria of AOSD [2]: he fulfilled all four major criteria (e.g., fever, arthralgia/arthritis, typical rash, and leukocytosis), and three of five minor criteria (e.g., sore throat, abnormal LDH concentration, and negative RF and ANA). The patient was treated with pulse of solumedrol (e.g., 1 g for 3 consecutive days) followed by daily oral prednisone 60 mg, that induced an immediate resolution of fever, and rapid respiratory improvement as well as resolution of arthritis and myalgia. CRP and neutrophilic leukocytosis decreased abruptly towards the normal values soon after the pulse-steroid therapy.
Fig. 1.
Kinetics of laboratory results.
2. Case 2
A 56-year-old woman was complaining of shortness of breath, chest pain, weakness, high spiking fever up to 39.5 c, sore throat, pain and swelling of metacarpo-phalangeal (MCP), proximal inter-phalangeal joints, and both knees and ankles, started seven days after receiving the second dose of the BNT162b2 mRNA vaccine against Covid-19 (Pfizer). She was initially diagnosed with probable acute bronchitis and was treated with amoxicillin/clavulanic acid. Despite treatment, the spiking fever persisted as well as the polyarthritis and she was hospitalized. On admission, the patient's body temperature was 38.7 °C, she had tachycardia (e.g., 110 bpm), and was dyspneic and on examination both lungs crepitations were found on auscultation. The small and large joints in the extremities were tender with local redness and a gentle pink maculopapular rash especially on the trunk and limbs.
Initial laboratory testing showed elevated CRP of 30.0 mg/dL, leukocytosis of 40.0 K/μL, a 10-fold increase of both hepatocellular and cholestatic liver enzymes, and hypoalbuminemia. On the next few days, the patient continued to complain of chest pain and dyspnea. The ECG disclosed non-specific T-wave changes in precordial lids, and a chest X-ray showed pleural effusion with normal heart contour. Serum troponin level was elevated (535 ng/L). TTE and percutaneous coronary intervention revealed normal left ventricular function and a mild pericardial effusion, and normal coronary arteries, respectively.
Serology and blood cultures for infectious causes were negative, as well as serology for rheumatology-related autoantibodies (e.g., RF, ACPA, ANA, ANCA). Serum ferritin level was remarkably elevated (49,149 ng/mL, reference 0-120 ng/mL).
Accordingly, the patient was diagnosed with AOSD in accordance with the Yamaguchi classification criteria of AOSD [2]: the patient met all four major criteria (fever, arthritis, rash and leukocytosis) and 3 minor criteria (sore throat, absence of RF factor and abnormal liver enzymes). Treatment with daily oral prednisone 60 mg was initiated (1 mg/kg) with immediate clinical improvement of the fever, dyspnea, rash and polyarthritis along with decrease in the serum levels of CRP and troponin decreased towards normal levels.
3. Discussion
These two cases of AOSD developing after the BNT162b2 mRNA Covid-19 Vaccine (Pfizer) represent a rare phenomenon. Indeed, both of our patients had some degree of myocarditis as manifested by transient elevation of troponin along with transient LV dysfunction, a recently recognized rare side effect of the COVID-19 vaccine [7]. Yet, our patients developed symptoms and signs involving the joins, skin, as well as inflammatory markers, which established the diagnosis of AOSD. In agreement, a single case of AOSD post Covid-19 vaccine has been reported recently [3].
AOSD is a rare inflammatory disease that affects subjects in the age range of 16-35 years [6]. In all three cases of AOSD-post COVID-19 vaccine, the subjects' age ranged between 40 and 60 years, and the patients responded quickly to treatment with corticosteroids without further complications. However, the patients reported here did not require advanced biologic treatments such as IL-1beta inhibitors or IL-6 receptor inhibitors, while the aforementioned case had been placed on anakinra [3].
COVID-19 infection induces inflammation, and in some cases, this could result in a hyperinflammatory response, wherein IL-1beta and IL-6 play a key role [4]. Similarly, it is suggested that the antigen, which is transcribed by the mRNA vaccines activate transiently both the innate and adaptive immune systems [5], and this antigen in the BNT162b2 mRNA COVID-19 vaccine is a foreign protein formulated in an immunostimulatory lipid nanoparticle [8]. Because both IL-1beta and IL-6 are elevated in AOSD as a result of aberrant activation of the innate immune system [6], it is therefore tempting to speculate that these inflammatory cytokines were induced in both of our patients in association with the BNT162b2 mRNA COVID-19 vaccine.
Altogether, AOSD-post COVID-19 vaccination is a rare and treatable side effect that should be recognized in adults. Early diagnosis and treatment would enable prompt recovery and the prevention of potential complications.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaguchi M., Ohta A., Tsunematsu T., Kasukawa R., Mizushima Y., Kashiwagi H., Kashiwazaki S., Tanimoto K., Matsumoto Y., Ota T., et al. Preliminary criteria for classification of adult Still’s disease. J. Rheumatol. 1992 Mar;19(3):424–430. 1578458 [PubMed] [Google Scholar]
- 3.Magliulo D., Narayan S., Ue F., Boulougoura A., Badlissi F. Adult-onset Still’s disease after mRNA COVID-19 vaccine. Lancet Rheumatol. 2021 Oct;3(10) doi: 10.1016/S2665-9913(21)00219-8. e680-e682. Epub 2021 Jul 22. PMID: 34316726; PMCID: PMC8298008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., Grinberg T., Auster O., Dagan N., Balicer R.D., Kornowski R. Myocarditis after Covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021 Oct 6 doi: 10.1056/NEJMoa2110737. 34614329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feist E., Mitrovic S., Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat. Rev. Rheumatol. 2018 Oct;14(10):603–618. doi: 10.1038/s41584-018-0081-x. PMID: 30218025; PMCID: PMC7097309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. Epub 2020 Mar 16. PMID: 32192578; PMCID: PMC7270045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019 Mar 27;10:594. doi: 10.3389/fimmu.2019.00594. PMID: 30972078; PMCID: PMC6446947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA Pfizer-BioNTech COVID-19 vaccine EUA Letter of Authorization reissued 02252021. 2020. https://www.fda.gov/media/144412/download