Abstract
Coronavirus disease (COVID-19) is an infection caused by the newly detected coronavirus, SARS-CoV-2. The majority of individuals will exhibit mild to moderate illness. Older individuals, and those suffering from co-existing diseases have a greater probability of experiencing a serious illness. Moreover, elderly patients have higher mortality rates than younger patients, especially those who are unvaccinated. Asymptomatic infection is mostly observed in individuals who are younger, as younger patients are more likely to exhibit a stronger immune response to the infection; aging is characterized by the decline immune function. In this article, a rare case of an unvaccinated 97-year-old woman is described who was admitted to Laiko General Hospital due to altered levels of consciousness, hypotension and a hematoma of the thoracic region, and tested positive for SARS-CoV-2 nucleic acid in a nasopharyngeal specimen and positive for IgG antibodies against the SARS-CoV-2 spike protein without a history of consistent manifestations, indicating a past asymptomatic infection.
Keywords: coronavirus, elderly, asymptomatic infection, humoral immune response, antibodies
Introduction
The newly discovered coronavirus SARS-CoV-2 causes the Coronavirus disease (COVID-19). Globally, as of October 21st, 2021, a total number of 241,411,380 confirmed cases of COVID-19, including 4,912,112 deaths have been documented (1). Most individuals who develop COVID-19 will experience mild to moderate manifestations and will recover without the need of specific treatment or hospitalization. Up to 20% of COVID-19 cases present with severe illness (2). In these cases, COVID-19 leads to a condition known as cytokine release syndrome, which affects and causes damage to different organs, including the lungs, heart and kidneys (3).
Asymptomatic infection is considered to be the positive detection of SARS-CoV-2 nucleic acid in an examined specimen from individuals with no symptoms of COVID-19, using reverse transcription-polymerase chain reaction (RT-PCR) (4). Current evidence demonstrates that several COVID-19 patients are asymptomatic, but can spread the virus. The management and risk assessment of these asymptomatic infections is one of the primary challenges posed to the current epidemic prevention and control strategies (5).
The proportion of asymptomatic cases varies in different studies. In a skilled nursing facility in Washington, 5.3% of 89 SARS-CoV-2 positive patients remained asymptomatic (6). In a study from Ningbo, China, among 2,147 close contacts of COVID-19 cases who were selected using prospective research methods, 132 were positive for SARS-CoV-2 nucleic acid, and 16.7% of these were completely asymptomatic (7). In another study from the United States, in which random sampling with 16,025 individuals of all ages were used, the prevalence of those with positive detection of SARS-CoV-2 nucleic acid was 1-6.9%, with 40% of them being asymptomatic (8).
Asymptomatic infections occurs mostly in younger individuals. A study from China reported that the asymptomatic individuals were mostly younger patients, and they had a lower possibility of developing severe respiratory infection (7). In a systematic review and meta-analysis of asymptomatic SARS-CoV-2 carriers, amongst 318 asymptomatic cases of COVID-19, 49.6% were children ≤18 years old, 30.3% were adults 19-50 years, and only 16.9% were elderly (≥51 years) (9). In another systematic review and meta-analysis of the epidemiological and radiographical characteristics of asymptomatic SARS-CoV-2 infections, infected children were more likely to be asymptomatic compared with adults and elderly individuals (10).
According to various studies, the mortality of elderly individuals with SARS-CoV-2 infection was higher than that of younger patients, and elderly patients with COVID-19 were more likely to progress to more severe disease (11,12). In addition, recent studies have shown that older unvaccinated adults have a greater possibility of being hospitalized or dying from COVID-19 than vaccinated ones (13).
Herein, a rare case of an extremely old unvaccinated woman, who experienced an asymptomatic SARS-CoV-2 infection and developed a sufficient antibody response, is reported.
Case report
A 97-year-old woman was admitted to our hospital for low blood pressure and altered levels of consciousness over the previous few hours. She had a medical history of dementia and immobility, arterial hypertension, ischemic stroke, hyperuricemia, gastrostomy, bilateral hip arthroplasty and a long-term urinary catheter with recurrent urinary tract infections. Her medications included escitalopram, mirtazapine, quetiapine, lercanidipine, enoxaparin and allopurinol.
Clinical evaluation revealed an afebrile patient with skin paleness and a large hematoma on the left side of the thoracic region, 15 cm in the greatest dimension. The patient had a Glascow Coma Scale score (14) of 9. Physical examination of the chest showed no abnormal findings on auscultation of the lungs and a systolic hearth murmur on aortic valve III/VI.
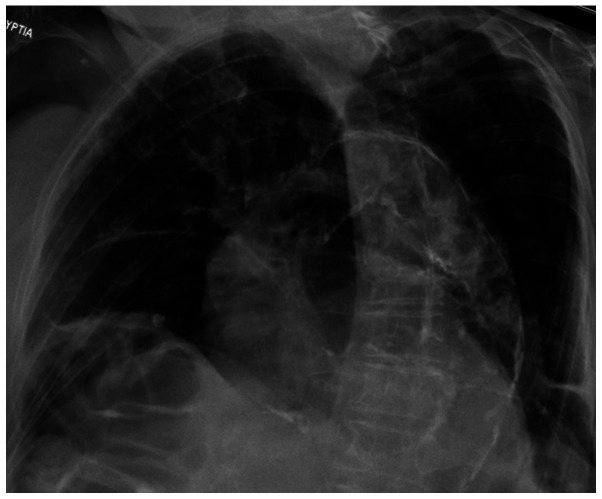
Blood pressure was 80/45 mmHg, heart rate was 110 beats per minute, oxygen saturation was 96% on room air and body temperature 36.6˚C. Electrocardiography revealed sinus tachycardia on admission. Chest X-ray revealed an increased cardiothoracic ratio, calcification of the aorta and thickening of the horizontal fissure without signs of consolidation or infiltrates (Fig. 1).
Figure 1.
Chest X-ray showing the increased cardiothoracic ratio, calcification of the aorta, and thickening of the horizontal fissure without signs of consolidation or infiltrates.
Laboratory investigation included complete blood cell count and standard biochemistry serum and urine parameters. Laboratory findings included hemoglobin (Hb) 5.8 g/dl (normal range, 12-16 g/dl), hematocrit 17.3% (normal range, 38-47%), red blood cell (RBC) count 2x106/µl (normal range, 4-6x106/µl), white blood cell (WBC) count 7.46x103/µl (normal range, 4-11x103/µl), platelet count 173x103/μl (normal 150-400x103/µl), serum glucose 87 mg/dl (normal range, 60-100 mg/dl), C-reactive protein (CRP) level 13.53 mg/l (normal range, <6 mg/l), prothrombin time 13.6 sec (normal range, 11-13 sec), activated partial thromboplastin time 33.4 sec (normal range, 29-40 sec), international normalized ratio 1.01 (normal range, 0.9-1.2) and d-dimer level 0.94 µg/ml (normal range, <0.5 µg/ml). The other blood biochemistry parameters were normal. Urinalysis revealed 40-60 WBCs per high power field (PHF) (normal range, 0-2 PHF), 10-20 RBCs PHF (normal range, 0-2 PHF), pH 6 (normal range, 4.5-8), specific gravity 1.006 (normal range, 1.005-1.025), and negative for nitrites and leukocyte esterase.
The patient was managed at the Emergency Department of Laiko General Hospital with the administration of crystalloid fluids via an intravenous infusion, resulting in elevation of blood pressure and rapid improvement of level of consciousness. For the safety of our patients and staff, our hospital is testing all patients admitted for SARS-CoV-2, regardless of whether they have signs or symptoms or not. The patient was tested for coronavirus, and was positive for SARS-CoV-2 nucleic acid in the examined nasopharyngeal sample using RT-PCR with a viral load of 11.707x106 copies/ml. The viral load was calculated based on the performance in the quantitative PCR. In this method, RNA was first transcribed into cDNA using a reverse transcriptase from total RNA or mRNA. The cDNA was then used as the template for the quantitative PCR reaction. A SARS-CoV-2 ELITe MGB automated, validated kit (cat. no. RTS170ING; CE/IVD; ELITechGroup) with the target genes RNA-dependent RNA polymerase (RdRp) and Open Reading Frame 8 (ORF8) was used. The kit included the following components: i) CoV-2 PCR mix which contained the specific primers and probes for a) the RdRp gene sequence of SARS-CoV-2, the probe RdRp was labelled with the AP593 fluorophore; b) The ORF8 gene sequence of SARS-CoV-2, the probe ORF8 was labelled with carboxyfluorescein fluorophore; and c) the human RNase P gene as the endogenous internal control. The CoV-2 PCR mix consisted of the buffer, magnesium chloride, nucleotide triphosphates, the stabilizers and the enzyme DNA polymerase with thermic activation (hot start). ii) RT EnzymeMix, which is an optimized and stabilized mixture of enzymes for reverse transcription, pre-aliquoted into test tubes. The ‘SARS-CoV-2 ELITe Standard’ kit (cat. no. STD170ING; ELITechGroup) was used for the detection and quantification of RNA of SARS-CoV-2. This kit contained four stabilized solutions of plasmid DNA at known titers, each aliquoted into two ready to use test tubes. The plasmid DNA solution contained the regions of the RdRp gene and ORF8 gene of SARS-CoV-2. Table I shows all the primer pair sequences that were used for SARS-CoV-2 reactions. For analysis of results, the 2-ΔΔCq method was used (15). The patient was unvaccinated for SARS-CoV-2, and was transferred to the COVID-19 unit, where she received 2 units of RBC transfusion.
Table I.
Sequences of the primers for SARS-CoV-2 PCR.
| Gene | Sequence, 5'-3' |
|---|---|
| RNA-dependent RNA polymerase | |
| Forward | CATCTCACTTGCTGGTTCCT |
| Reverse | CCTTAATAGTCCTCACTTCTCTC |
| Open Reading Frame 8 | |
| Forward | TGATGATACTCTCTGACGATGCTGTCTCAGTCCAACATTTTGCTTCAGA |
| Reverse | ATGCATCTCAAGGTCTAGTG |
The patient had no obvious symptoms or signs of COVID-19 or radiographical findings. According to her caregiver, the old woman had not presented with fever, cough or other symptoms consistent with COVID-19 in the last month. In addition, she had not presented with worsening of cognitive symptoms lately or other symptoms, such as diarrhea, vomiting or nausea that could represent an atypical COVID-19 presentation. Her caregiver and all close contacts of the patient also denied having symptoms attributable to COVID-19 recently, and were tested for SARS-CoV-2 with RT-PCR, and all were negative in their nasopharyngeal samples. The caregiver also denied that the patient had fallen, which could be a possible explanation for the presence of the hematoma.
The patient exhibited stability in their hematocrit and gradual resolution of the hematoma on the second day of hospitalization. On the third day of hospitalization, in the absence of symptoms and any SARS-CoV-2 positive close contacts, it was decided to perform an antibody test for COVID-19 in the patient. The antibody test for SARS-CoV-2 spike protein was performed using blood serum and the chemiluminescence method (SARS-CoV-2 Spike RBD Antibody kit; cat. no. MAB108271-SP; R&D Systems, Inc.). It resulted in positive detection of IgG antibodies with a titer of 394,00 BAU/ml (<33.8 BAU/ml, negative; >33,8 BAU/ml, positive) and IgM antibodies with a titer of 1,41 AU/ml (<1,1 AU/ml, negative; >1,1 AU/ml, positive), indicating that the old woman had already experienced an asymptomatic SARS-CoV-2 infection. IgA antibodies were not determined, nor where anti-N SARS-CoV-2 antibodies, as this was certainly a natural infection, given that the patient did not have a history of vaccination for SARS-CoV-2.
The patient was discharged on the fourth day of the hospitalization. She had an uneventful 3 month follow-up and she is still alive. The patient was tested for SARS-CoV-2 with RT-PCR, providing a negative result in their nasopharyngeal samples 3 months after hospitalization.
Ethical approval for this case was obtained from the Research Ethics Committee of Laiko General Hospital (approval no. 14750). Written informed consent was obtained from the daughter of the patient for publication of this case report and accompanying images, as the patient was too old and suffered from dementia.
Discussion
The present case was unique for three reasons. Firstly, the patient was a very old woman with an asymptomatic SARS-CoV-2 infection. Studies have demonstrated that asymptomatic infections are more frequent in populations of younger and middle-aged individuals with a good performance status and without comorbidities (16,17). The COVID-19 pandemic has caused significant mortality in populations regarded to be at risk, such as elderly individuals, especially those who are health care facility residents. The vulnerability of this population is associated with physiological aspects of aging, including the reduced effectiveness of the immune system, resulting in morbidity and mortality from infections. Advancing age and SARS-CoV-2 infection worsen fragility, especially for hospitalized older adults, who tend to present with a more accentuated clinical course compared to the classic symptoms of the disease (18).
Mori et al (19) in their study compared COVID-19 infection between young and elderly patients, and reported that although the extent of lung involvement did present a significant difference between the two groups, elderly patients had a greater probability of having a severe clinical course, possibly due to difference in lung anatomy, muscle atrophy, impaired airway clearance, reduced lung reserve and reduced ability to defend against infections. Liu et al (11) concluded that the mortality rates of elderly patients with the novel coronavirus infection were higher than those of younger and middle-aged patients, and the proportion of older patients with high grades of pneumonia severity index score was significantly higher than that of younger ones. The prevalence of the common fatal complications of COVID-19, including shock, acute cardiac injury and acute respiratory distress syndrome, in elderly patients is also higher compared with younger patients according to other studies (20,21). Increased expression of angiotensin converting enzyme (ACE)-2 in patients receiving ACE inhibitors and angiotensin II receptor blockers, as well as previous exposure to circulating coronaviruses with low neutralizing capacity to SARS-CoV-2 may all contribute to the elderly individuals' increased susceptibility to this infection (22).
Secondly, the patient had a favorable course of SARS-CoV-2 infection, despite having a high viral load. According to a study by Wang et al (16), an older age is correlated with higher SARS-CoV-2 load, and current studies have reported that a high initial viral load is related to death (23,24). Increasing evidence has shown that the SARS-CoV-2 viral load is linked to the risk of COVID-19 progression and a poorer prognosis (25). However, whether the unfavorable prognosis of elderly COVID-19 patients is related to a higher viral load remains to be determined. It is notable that a high viral load has also been associated with elevated levels of cytokines, such as TNF-α, IFN-γ, IL-2, IL-4, IL-6, IL-10 and CRP, all contributing to hyper-inflammation (26) and serum inflammatory factors are positively correlated with the production of specific antibodies in COVID-19 patients, according to a study by Zheng et al (27). In the present case, the patient had a high viral load, despite having been infected several days ago, as indicated by low titers of IgM antibodies. It has been shown that SARS-CoV-2 RNA fragments may integrate into the human genome (28). This finding may explain the persistence of positive RT-PCR results in certain individuals.
Thirdly, the patient developed an antibody response to SARS-CoV-2 infection despite her advanced age. Aging is accompanied with a remodeling of the immune system, possibly due to chronic antigen exposure, altered telomerase activity, dysfunction of mitochondria, a defective autophagy and ubiquitin/proteasome system, and alterations in the gut microbiota composition (29). Elderly patients with SARS-CoV-2 infection frequently present with a significant dysregulation of pro-inflammatory cytokines, which may lead to a worse outcome (30). According to a study by Rydyznski Moderbacher et al (31), coordination of the SARS-CoV-2 antigen-specific responses, which is related to milder disease, is interrupted in elderly individuals (31). On the other hand, in a retrospective study of 1,071 adults with symptomatic SARS-CoV-2 infection Bag Soytas et al (32), the IgG antibody titer was higher in patients >80 years old. However, the patients were all symptomatic and the high IgG antibody titers were correlated with the severity of the disease.
Another noteworthy issue is that the patient presented with severe anemia, which was attributed to the large hematoma in the thoracic region. Anemia affects mostly the elderly and frail patients, negatively influencing their quality of life. However, anemia has not been associated with worse outcomes of SARS-CoV-2 infection (33).
In conclusion, the present report describes the rare case of an asymptomatic SARS-CoV-2 infection in an unvaccinated extremely old woman. Elderly individuals can be asymptomatic carriers SARS-CoV-2 with high viral loads, contributing to the transmission and spread of the novel coronavirus virus. Moreover, they can develop a sufficient antibody response, despite the absence of symptoms and their advanced age.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
AB, CS and PP conceptualized the case. VEG, AGa, NG, CD, AGk, KM and SC made substantial contributions to the conception and design of the study. NT and DAS analyzed and interpretated the data. PS prepared the figure. All authors contributed to manuscript revision and approved the final version of the manuscript. VEG and AGk confirm the authenticity of all the data.
Ethics approval and consent to participate
Ethical approval for this case was obtained from the Research Ethics Committee of Laiko General Hospital (approval no. 14750).
Patient consent for publication
Written informed consent was obtained from the daughter of the patient for publication of this case report and accompanying images, as the patient was too old and suffered from dementia.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1. World Health Organization (WHO): WHO Coronavirus (COVID-19) Dashboard. WHO, Geneva, 2021. https://covid19.who.int/. [Google Scholar]
- 2.Georgakopoulou VE, Garmpis N, Damaskos C, Valsami S, Dimitroulis D, Diamantis E, Farmaki P, Papageorgiou CV, Makrodimitri S, Gravvanis N, et al. The Impact of peripheral eosinophil counts and eosinophil to lymphocyte ratio (ELR) in the clinical course of COVID-19 Patients: a retrospective study. In Vivo. 2021;35:641–648. doi: 10.21873/invivo.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO): COVID-19 Clinical management: living guidance. WHO, Geneva, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. Accessed January 25, 2021. [Google Scholar]
- 5.Chen Z, Wang B, Mao S, Ye Q. Assessment of global asymptomatic SARS-CoV-2 infection and management practices from China. Int J Biol Sci. 2021;17:1119–1124. doi: 10.7150/ijbs.59374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spicer K, Bardossy AC, Oakley LP, et al. Public Health-Seattle and King County and CDC COVID-19 Investigation Team: Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, Ma H, Chen W, Lin Y, Zheng Y, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng CX. The global battle against SARS-CoV-2 and COVID-19. Int J Biol Sci. 2020;16:1676–1677. doi: 10.7150/ijbs.45587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syangtan G, Bista S, Dawadi P, Rayamajhee B, Shrestha LB, Tuladhar R, Joshi DR. Asymptomatic SARS-CoV-2 carriers: a systematic review and meta-analysis. Front Public Health. 2021;8(587374) doi: 10.3389/fpubh.2020.587374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Zhu C, Yan D, Liu H, Li D, Zhou Y, Fu X, Wu J, Ding C, Tian G, et al. The epidemiological and radiographical characteristics of asymptomatic infections with the novel coronavirus (COVID-19): A systematic review and meta-analysis. Int J Infect Dis. 2021;104:458–464. doi: 10.1016/j.ijid.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52:154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention (CDC): COVID-19 Risks and Vaccine Information for Older Adults. CDC, Atlanta, GA, 2021. https://www.cdc.gov/aging/covid19/covid19-older-adults.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Folder-adults.html. Last reviewed August 2, 2021. [Google Scholar]
- 14.Yates DW. ABC of major trauma. Scoring systems for trauma. BMJ. 1990;301:1090–1094. doi: 10.1136/bmj.301.6760.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Liu Y, Liu L, Wang X, Luo N, Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Chen X, Wang L, Zheng R. Clinical Characteristics of 33 Asymptomatic COVID-19 Infections in Wuhan, China. J Infect Dev Ctries. 2020;14:1252–1255. doi: 10.3855/jidc.13222. [DOI] [PubMed] [Google Scholar]
- 18.Alves VP, Casemiro FG, Araujo BG, Lima MAS, Oliveira RS, Fernandes FTS, Gomes AVC, Gregori D. Factors associated with mortality among elderly people in the COVID-19 pandemic (SARS-CoV-2): a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(8008) doi: 10.3390/ijerph18158008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori H, Obinata H, Murakami W, Tatsuya K, Sasaki H, Miyake Y, Taniguchi Y, Ota S, Yamaga M, Suyama Y, et al. Comparison of COVID-19 disease between young and elderly patients: Hidden viral shedding of COVID-19. J Infect Chemother. 2021;27:70–75. doi: 10.1016/j.jiac.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, Wei J, Gong Z, Zhou C, Yu H, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26:767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, Zhan LY, Jia Y, Zhang L, Liu D, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21(100331) doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peron JPS, Nakaya H. Susceptibility of the elderly to SARS-CoV-2 infection: ACE-2 overexpression, shedding, and antibody-dependent enhancement (ADE) Clinics (São Paulo) 2020;75(e1912) doi: 10.6061/clinics/2020/e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu CM, Poon LL, Cheng VC, Chan KS, Hung IF, Wong MM, Chan KH, Leung WS, Tang BS, Chan VL, et al. Initial viral load and the outcomes of SARS. CMAJ. 2004;171:1349–1352. doi: 10.1503/cmaj.1040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Sun S, Shi Y, Wang H, Zhao R, Sheng J. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit Care. 2020;24(170) doi: 10.1186/s13054-020-02893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenoy S. SARS-CoV-2 (COVID-19), viral load and clinical outcomes; lessons learned one year into the pandemic: A systematic review. World J Crit Care Med. 2021;10:132–150. doi: 10.5492/wjccm.v10.i4.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng M, Gao Y, Liu S, Sun D, Yang F, Zong L, Zhang M, Tian Z, Xu Y, Sun H. Serum inflammatory factors are positively correlated with the production of specific antibodies in coronavirus disease 2019 patients. Cell Mol Immunol. 2020;17:1180–1182. doi: 10.1038/s41423-020-00551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Richards A, Khalil A, Wogram E, Ma H, Young RA, Jaenisch R. SARS-CoV-2 RNA reverse-transcribed and integrated into the human genome. bioRxiv: doi: 10.1101/2020.12.12.422516. [Google Scholar]
- 29.Cunha LL, Perazzio SF, Azzi J, Cravedi P, Riella LV. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol. 2020;11(1748) doi: 10.3389/fimmu.2020.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, Liu W, Zhu Y, Lin Q, Mao L, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(e137799) doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bag Soytas R, Cengiz M, Islamoglu MS, Uysal BB, Ikitimur H, Yavuzer H, Yavuzer S. Does the COVID-19 seroconversion in older adults resemble the young? J Med Virol. 2021;93:5777–5782. doi: 10.1002/jmv.27106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergamaschi G, Borrelli de Andreis F, Aronico N, Lenti MV, Barteselli C, Merli S, Pellegrino I, Coppola L, Cremonte EM, Croce G, et al. Anemia in patients with Covid-19: Pathogenesis and clinical significance. Clin Exp Med. 2021;21:239–246. doi: 10.1007/s10238-020-00679-4. Internal Medicine Covid-19 Collaborators. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.