Figure 5. The HCRT neuropeptide pathway controls confinement-induce sighing through Nmb neurons.
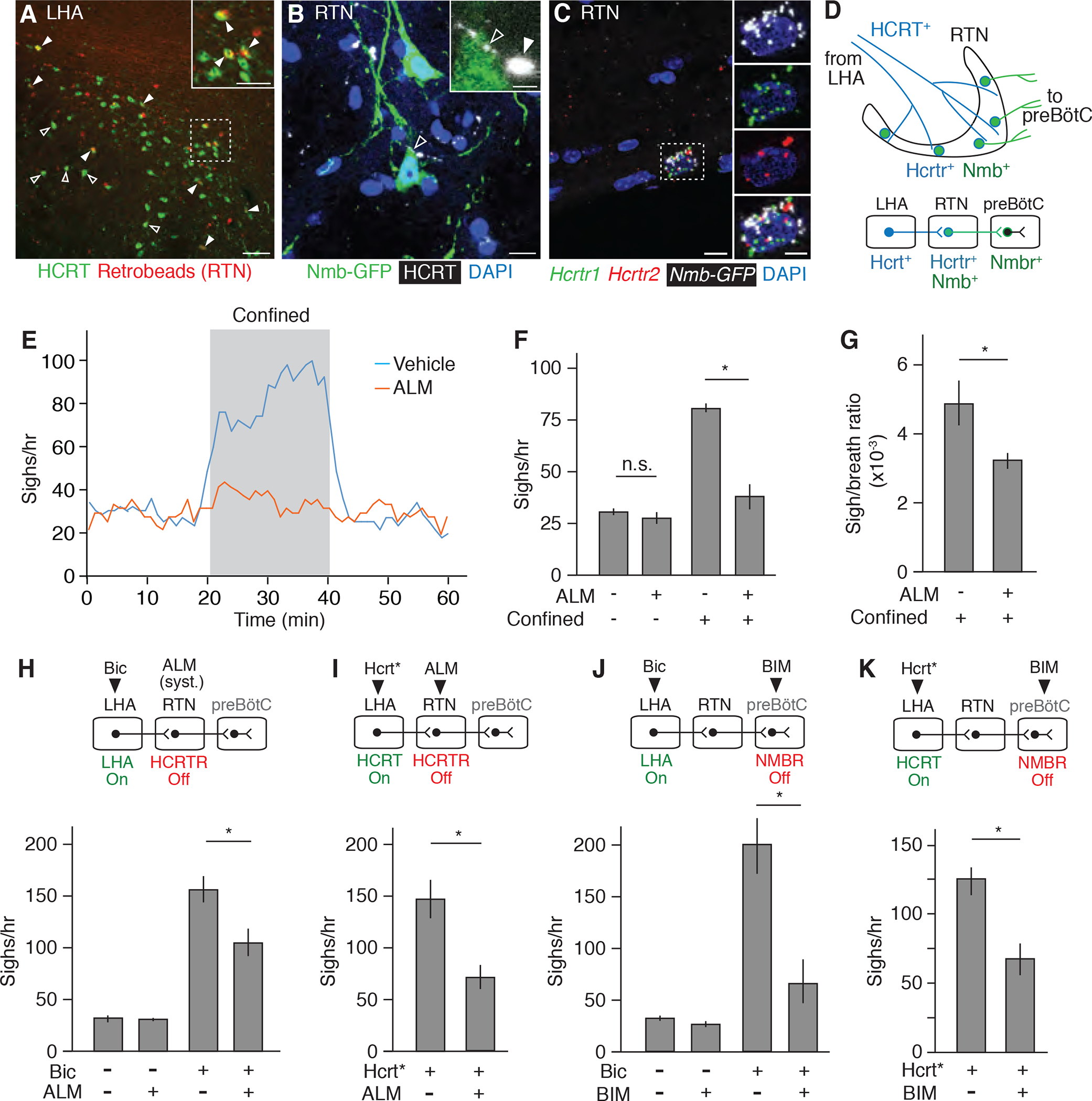
(A) LHA brain slice of adult mouse injected in RTN with fluorescent retrograde tracer (retrobeads, red) then immunostained for HCRT1 (green) 5 days later. Arrowheads, HCRT1+ neurons co-stained with retrobeads, indicating projection to RTN; the retrobead (red) signal for some co-stained neurons (open arrowheads) is obscured by the strong HCRT signal (green) in image shown but readily detected when red and green channels are split (not shown). Inset, close-up of boxed region. Scale bars, 100 um (50 um, inset). (B) RTN region of adult Nmb-GFP BAC transgenic mouse immunostained for GFP (green) and HCRT1 (white pseudocolor). Open arrowhead, HCRT1 puncta in axon directly abutting Nmb-GFP positive neuron (green); inset shows close-up. Filled arrowhead, HCRT1 puncta close though not abutting Nmb-GFP positive neuron (green). Scale bar, 10 um (2 um, inset). (C) Multiplex single molecule in situ hybridization (RNAscope) of RTN region probed for Hcrtr1 (green), Hcrtr2 (red) and Egfp (white pseudocolor) transcripts. Note isolated Nmb-GFP+ neuron co-expressing both receptors (boxed, enlarged in right panels that show split channels). Scale bar, 10 um (5 um, inset). (D) Diagram (upper) and schematic (lower) of A to C showing HCRT+ processes (blue) from LHA projecting to Nmb-expressing neurons in RTN (green) that co-express Hcrt receptors Hcrtr1 and Hcrtr2 and project to Nmbr-expressing neurons in preBötC. (E) Sigh rate before, during, and after tube confinment of awake, behaving wild-type mice (bin 4 min, slide 1 min) gavage fed with 100 mg/kg HCRTR antagonist almorexant (ALM, red; n=7) or vehicle (blue; n=8) 2 hours before plethysmography. Grey, confinement period. (F and G) Quantification of e showing sigh rate (F) and sigh-to-eupneic-breath ratio (G) of ALM-treated (+, n=7) and vehicle control (−, n=8) animals before (−) and during (+) confinement. Data as mean +/−SEM; *, p<0.05 (unpaired t-test). (H) Sigh rate before (−) and after (+) stereotactic bicuculline (Bic) injection to de-repress (activate) LHA of anesthetized mice treated systemically with ALM (+) or vehicle control (−) 2 hours before plethysmography. Experimental scheme is diagrammed above graph. Note reduction in LHA-induced sighing by systemic (syst.) HCRTR antagonism. n=6 per condition. (I) Sigh rate during optogenetic LHA Hcrt-IRES-Cre neuron activation (Hcrt*, Fig. 4C) of anesthetized mice injected in RTN with ALM (+, n=7) or vehicle control (−, n=5). Note reduction in Hcrt neuron-induced sighing by HCRTR antagonism in RTN. (J) Sigh rate before (−) and after (+) bicuculline injection to de-repress LHA of anesthetized mice with NMBR antagonist BIM23042 (+, n=7) or vehicle (−, n=6) injected in preBötC. Note reduction in LHA-induced sighing by NMBR antagonism in preBötC. (K) Sigh rate during optogenetic LHA Hcrt-IRES-Cre neuron activation of anesthetized mice with BIM23042 (+) or vehicle (−) injected into preBötC. Note reduction in HCRT neuron-induced sighing by NMBR antagonism in preBötC. n=6 per condition. For H to K, mean +/−SEM; *, p<0.05 (unpaired t-test).
