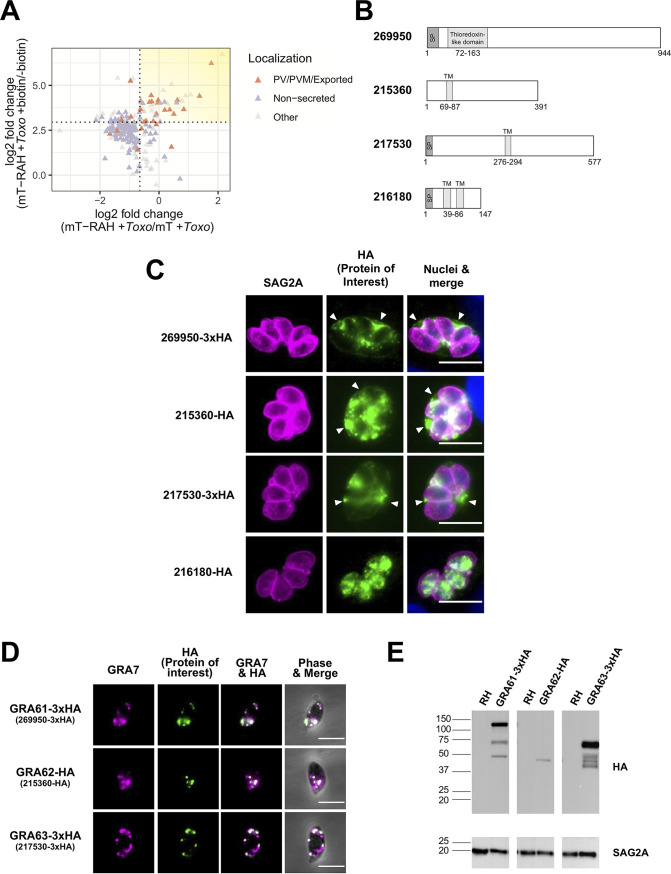
FIG 5.
The newly identified Toxoplasma proteins 269950 (GRA61), 215360 (GRA62), and 217530 (GRA63) localize to the Toxoplasma parasitophorous vacuole in infected cells and derive from the dense granules. (A) Two-dimensional plot showing log2 fold changes between miniTurbo-RAH and miniTurbo-infected samples (x axis) and log2 fold changes between +biotin and –biotin miniTurbo-RAH samples (y axis). Toxoplasma proteins known to be exported or PV/PVM-localized or putatively nonsecreted (see Materials and Methods) are indicated by orange and purple symbols, respectively. Dotted lines indicate the optimal threshold for separation of known PV/PVM/exported versus nonsecreted Toxoplasma proteins. Yellow shading indicates the position on the plot of the proteins of interest described in Table 1. (B) Schematic representations of the indicated proteins showing predicted N-terminal signal peptides (SP), predicted transmembrane domains (TM), and predicted homology to conserved domains from ToxoDB.org (version 53). (C) Representative immunofluorescence microscopy images of endogenously tagged HA-protein (269950-3xHA and 217530-3xHA) or protein ectopically expressed under its native promoter (215360-HA and 216180-HA). The tachyzoite single clones expressing HA-tagged proteins of interest were allowed to infect HFFs for 20 h before the infected monolayers were fixed with methanol. The corresponding tagged proteins were detected with rat anti-HA antibodies (green), while all tachyzoites were detected with rabbit anti-SAG2A antibodies (magenta). Host and parasite nuclei were stained with DAPI (blue). Arrowheads indicate vacuoles with HA-staining outside the parasites. Scale bar is 10 μm. (D) Representative immunofluorescence microscopy images of free tachyzoites expressing endogenously HA-tagged protein (269950-3xHA and 217530-3xHA) or protein ectopically expressed under its native promoter (215360-HA). Staining was with rabbit anti-GRA7 antibodies (magenta) and rat anti-HA antibodies (green) with the data superimposed on phase contrast images of the parasites themselves in the right-most panels. Colocalization is apparent as white coloring in the merged images. Scale bar is 5 μm. (E) Western blotting of HA-tagged proteins. Lysates of HFFs infected with parasite single clones expressing GRA61/62/63 were resolved by SDS-PAGE, blotted, and probed with rat anti-HA antibodies to detect the HA-tagged proteins. Rabbit anti-SAG2A staining was used as a loading control for total parasite protein. Approximate migration of a ladder of size standards (sizes in kDa) is indicated.