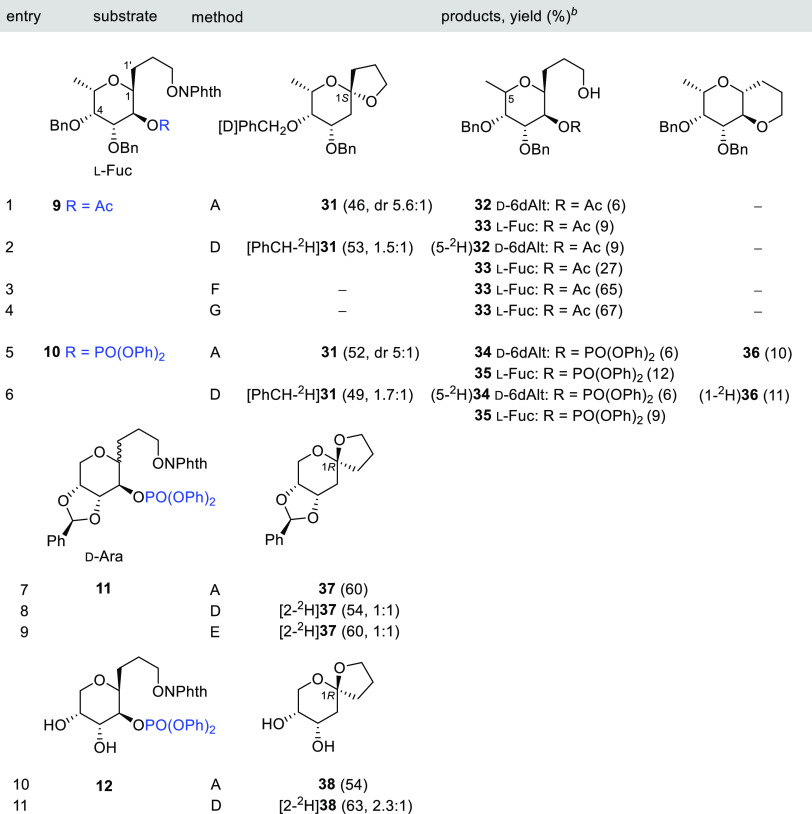
Table 3. 1,5-HAT–S–T Sequence in 3-C-(α-l-Fucp)- and 3-C-(d-Arap)1-propoxyphthalimides 9–12a.
Reagents and conditions: method A: n-Bu3SnH (1 equiv), AIBN (0.1 equiv), PhCH3 (0.013 M), reflux; method D: n-Bu3SnD (1 equiv), AIBN (0.1 equiv), PhCH3 (0.013 M), reflux; method E: n-Bu3SnD (1 equiv), BF3•Et2O (0.2 equiv), AIBN (0.1 equiv), PhCH3 (0.013 M), reflux; method F: Hantzsch ester (1.1 equiv), fac-Ir(ppy)3 (0.01 equiv), THF (0.007 M), rt, blue LED; and method G: Hantzsch ester (0.37 equiv/h), fac-Ir(ppy)3 (0.01 equiv), THF (0.007 M), rt, blue LED.
Values in parentheses are isolate yields; deuterium incorporation (2H/1H) is included in partially labeled compounds. dr = diastereomeric ratio; only the major isomer is shown.