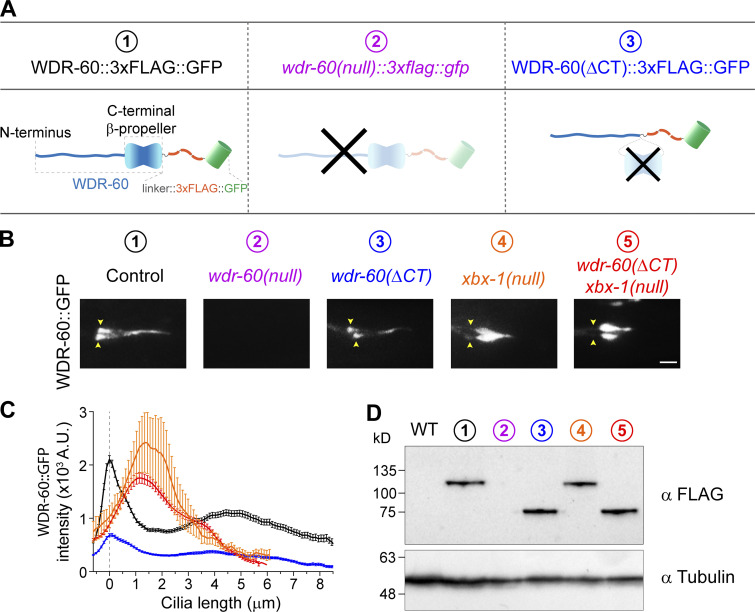
Figure 2.
Truncation of the β-propeller domain reduces, but does not abolish, entry of WDR-60 into cilia. (A) Schematic representation of WDR-60 tagging with 3xFLAG::GFP in control, wdr-60(tm6453) null, and truncated wdr-60 knock-in strains: (1) full-length WDR-60, composed of an NT disordered region and a CT β-propeller domain; (2) wdr-60(tm6453), predicted to be a null mutant; (3) wdr-60(ΔCT), expected to produce a protein composed of the WDR-60 NT fused to the 3xFLAG::GFP tag (lacking the β-propeller). (B) Phasmid cilia of each wdr-60 knock-in strain as indicated. Yellow arrowheads indicate the ciliary base. Note that no GFP signal is detected in the wdr-60(tm6453)::3xflag::gfp strain. Scale bar, 2 µm. (C) Quantification of GFP signal intensity distribution along the cilium in wdr-60 mutants shown in B (n ≥ 55 cilia). Graph is shown as mean ± SEM. (D) Western blot of extracts from wild-type and wdr-60 knock-in strains using an anti-FLAG antibody. The predicted sizes are 105.6 kD for WDR-60::3xFLAG::GFP and 63.2 kD for WDR-60(ΔCT)::3xFLAG::GFP truncation. No signal is detected in wdr-60(tm6453)::3xflag::gfp extracts, demonstrating that this is indeed a null strain. α-Tubulin was used as a loading control. Source data are available for this figure: SourceData F2.