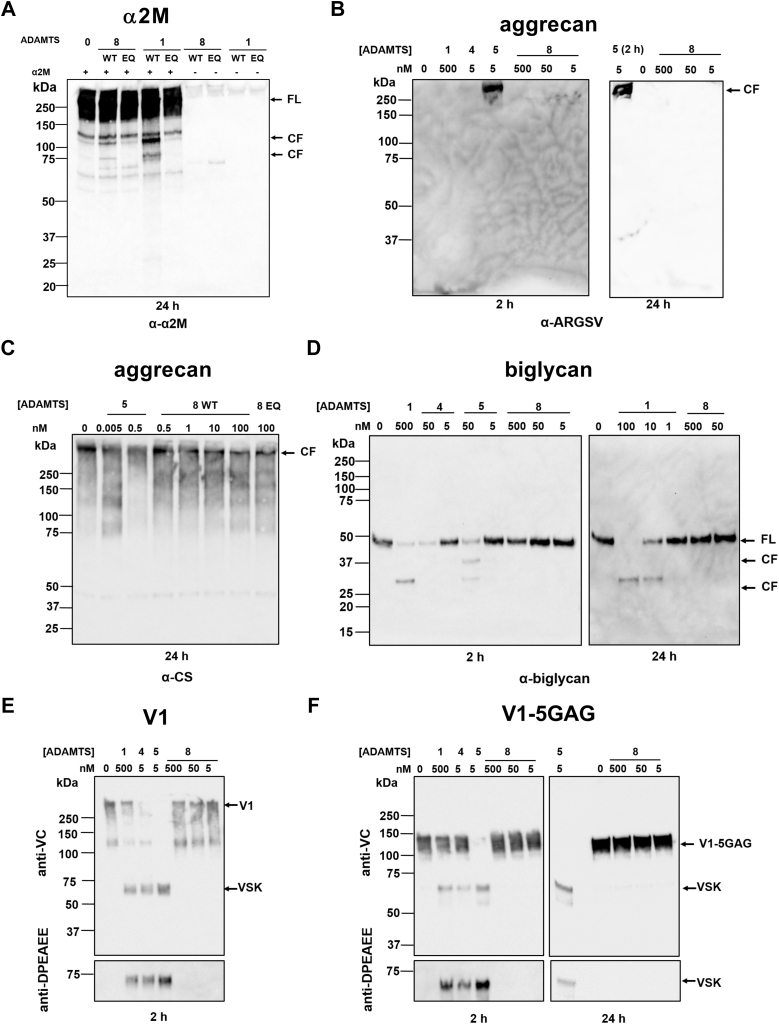
Figure 5.
Proteolytic activity of ADAMTS8.A, ADAMTS8 cleaves α2-macroglobulin. α2M (250 nM) was incubated with either ADAMTS8 or ADAMTS1 (250 nM) for 24 h. Samples were subjected to 4 to 12% SDS-PAGE and probed with polyclonal anti-α2M antibodies. Full-length (FL) substrates and specific cleavage fragments (CF) are indicated. All reactions were performed with the WT or EQ form of each enzyme. B, activity of ADAMTS1 (500 nM), ADAMTS4 (5 nM), ADAMTS5 (5 nM), and ADAMTS8 (5–500 nM) against bovine aggrecan (667 nM). Cleavage fragments generated after either 2 or 24 h digestion (as indicated) were detected using the anti-ARGSV neoepitope antibody BC3. C, activity of ADAMTS5 (0.005–0.5 nM) and ADAMTS8 (0.5–100 nM) against aggrecan (667 nM) after 24 h digestion. Activity of 100 nM ADAMTS8 EQ is shown for comparison. Bands were detected by the antibody 2B6, recognizing the CS stubs remaining following treatment of aggrecan with chondroitinase ABC. D, activity of ADAMTS1 (1–500 nM), ADAMTS4 (5–50 nM), ADAMTS5 (5–50 nM), and ADAMTS8 (5–500 nM) against biglycan (2 μM) was investigated after 2 or 24 h digestion (as indicated) using polyclonal antibodies against biglycan. Full-length (FL) substrates and specific cleavage fragments (CF) are indicated. E and F, activity of ADAMTS1 (500 nM), ADAMTS4 (5 nM), ADAMTS5 (5 nM), and ADAMTS8 (5–500 nM) against versican V1 (E) and V1-5GAG (F) (each at 100 nM) after 2 and 24 h digestions. Bands were detected using anti-VC and anti-DPEAEE antibodies. All digestions were performed at 37 °C. Representative blots from n = 3 independent replicates are presented. VSK, versikine.