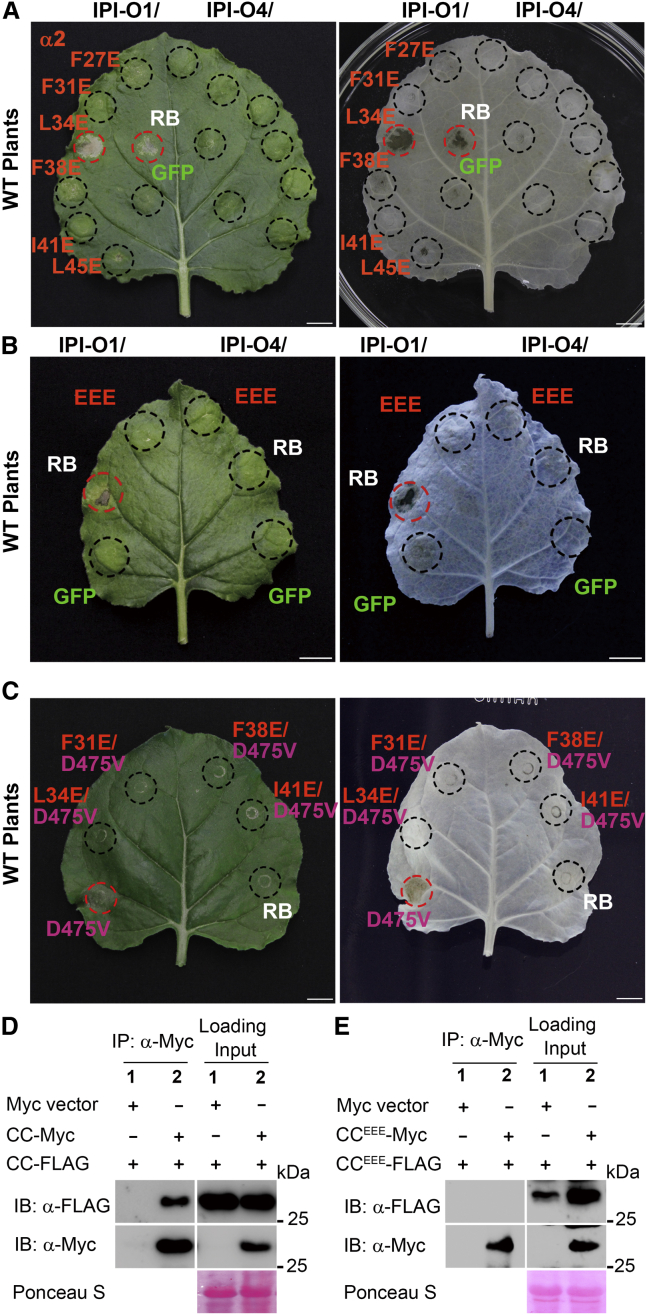
Figure 3.
Mutations in the heptad repeats of RB abolish its function and disrupt self-association of the RB CC domain
(A) RB and its variants carrying mutations in the heptad repeats from the second α helix were each co-expressed with Myc-IPI-O1 or Myc-IPI-O4 in N. benthamiana.
(B) RB and the F31E/L34E/I41E triple mutant (EEE) were each co-expressed with Myc-IPI-O1 or Myc-IPI-O4 in N. benthamiana.
(C) Cell death-inducing activity of the RB variants carrying the autoactive D475V mutation in the MHD motif in combination with mutations in the heptad repeats of RB CC. Cell death induced at 48 hpi was visualized before (left) and after ethanol destaining (right). The infiltrated area is shown with a black circle and HR with a red circle. Scale bars correspond to 1 cm. The experiments in (A, B, and C) were repeated at least six times with similar results.
(D) The RB CC domain associates with itself in vivo. Total proteins were extracted from N. benthamiana plants expressing RB CC-3×FLAG and RB CC-4×Myc. Immunoprecipitation was performed with an anti-Myc antibody, and immunoblots were probed with anti-Myc or anti-FLAG antibodies.
(E) The CC domain carrying the triple F31E/L34E/I41E mutation (CCEEE) is unable to self-associate in vivo. Total proteins were extracted from N. benthamiana plants expressing CCEEE-3×FLAG and CCEEE-4×Myc. Immunoprecipitation was performed with an anti-Myc antibody, and immunoblots were probed with anti-Myc or anti-FLAG antibodies. Ponceau S staining of immunoblots served as a loading control. The experiments in (D and E) were repeated three times with similar results.