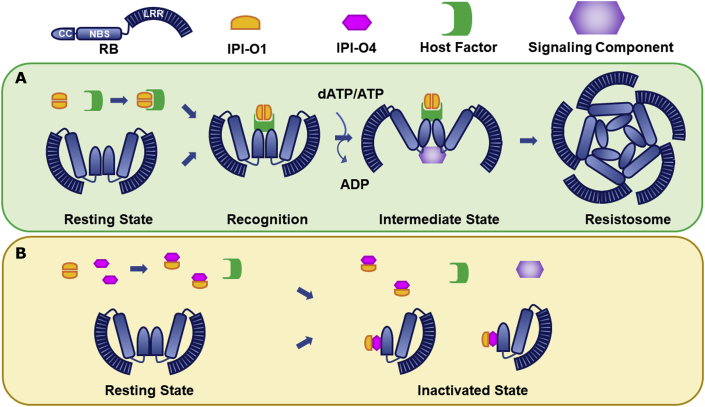
Figure 7.
A working model of NLR receptor RB-mediated recognition of its cognate effector IPI-O1 and its suppression by the virulence effector IPI-O4.
(A) In the resting state, RB self-associates via the CC domain, adopting a closed and inactive conformation. When IPI-O1 is present, it is recognized by RB via the CC domain, probably through an unidentified host factor, and RB adopts an open conformation, potentially leading to the recruitment of signaling components to assemble an active resistosome complex.
(B) When IPI-O4 is present, it perturbs the self-association of both RB and IPI-O1 and enhances the RB/IPI-O1 interaction, thereby probably preventing the formation of interaction interfaces that are required for recruitment of signaling components and assembly of the resistosome.