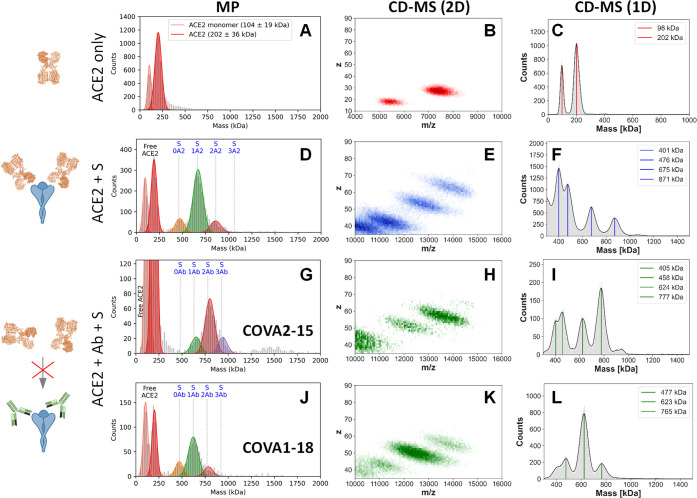
Figure 5.
Substoichiometric Ab binding to the S-trimer is sufficient to neutralize receptor binding. (A–C) MP and CD-MS histograms of ACE2 alone, revealing the dimeric nature of the utilized ACE2 construct and (D–F) ACE2 binding to the S-trimer. These results show that ACE2 is largely dimeric, and only the ACE2-dimer binds to S-trimer, whereby the S-trimer can accommodate either one or two ACE2. (G–L) MP and CD-MS histograms of ACE2 binding to the S-trimer following preincubation with either (G–I) COVA2-15 or (J–L) COVA1-18. The observed mass shifts of ∼150 kDa (and not 200 kDa) indicate that both Abs fully prevent ACE2 binding to the S-trimer. Mixing ratios of 4:1 and 4:4:1 (ACE2-dimer:S-trimer and Ab:ACE2-dimer:S-trimer, respectively) were used for the CD-MS experiments, while 1:1 and 3:1:1 were used for the MP experiments. Note the similarities between the data presented in panel G and Figure 2A, and panel J and Figure 2B.