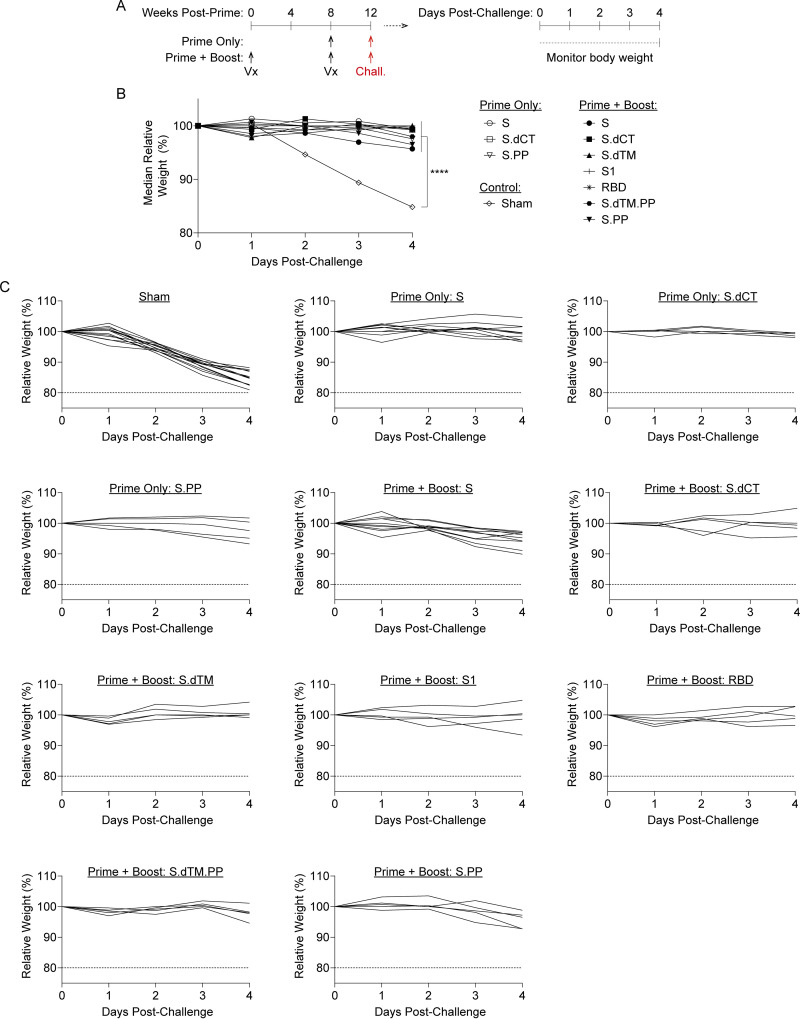
FIG 3.
RhAd52 vaccines protect from clinical disease following mouse-adapted SARS-CoV-2 challenge. (A) Groups of mice were immunized with either a prime and boost or a prime only of 109 viral particles (VPs) of RhAd52 candidate vaccines following the indicated timeline. At week 12, mice were challenged with 104 PFU of MA10 SARS-CoV-2 via the intranasal route. After challenge, a subset of mice was followed through day 4 postchallenge to monitor for signs of clinical disease. (B) Relative body weight following MA10 SARS-CoV-2 challenge in mice immunized with the indicated vaccine regimens. The median value of each group is displayed. ****, P < 0.0001 (results of a one-way ANOVA followed by Dunnett’s multiple-comparison test, comparing vaccinated groups to the sham control group). (C) Traces of relative body weight in individual mice, immunized with the indicated RhAd52 vaccine regimen, following challenge. For panels B and C, data are pooled from two similar experiments. n = 5 mice/group for all regimens, with the exception of RhAd52.S prime + boost (n = 10), RhAd52.S prime only (n = 10), and sham (n = 13).