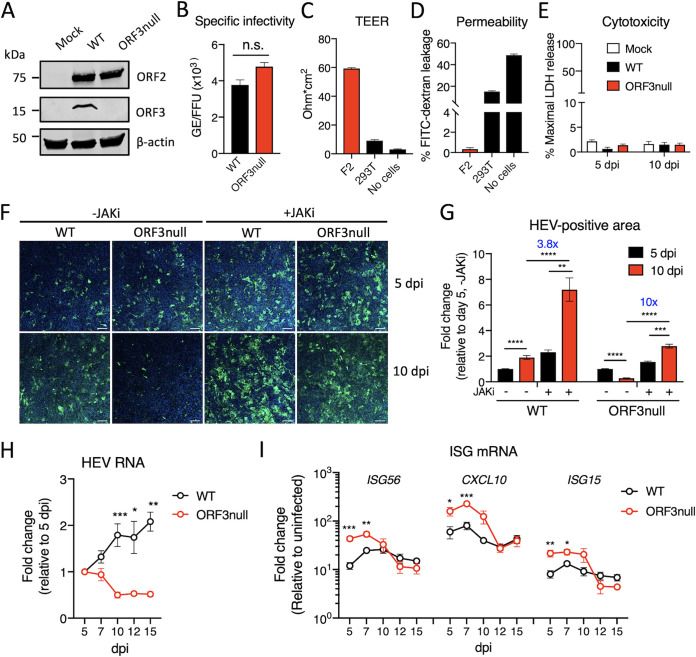
FIG 1.
ORF3 is required for efficient HEV growth in polarized human hepatocytes. (A) Western blots of HEV ORF2 and ORF3 proteins in HepG2/C3A cells 5 days after transfection with wild-type (WT) or ORF3null HEV RNA. β-Actin served as the loading control. (B) Specific infectivity of WT and ORF3null virions recovered from HEV RNA-transfected cells. HEV genome equivalents (GE) were determined by qRT-PCR. ORF2-positive focus-forming units (FFU) were determined in F2 cells (a subclone of HepG2/C3A cells) by immunofluorescence assays at 5 days postinoculation. n.s., not significant. (C and D) Transepithelial electrical resistance (TEER) (C) and permeability (D) of F2 or 293T cells grown on a porous membrane (pore size, 3 μm) for 18 days. Data are shown as means ± standard errors of the means (SEM) from 2 independent experiments, each with duplicate wells. (E) Polarized F2 cells were inoculated with WT or ORF3null HEV (103 GE per cell). Lactate dehydrogenase (LDH) levels in the basolateral culture medium were measured at 5 and 10 days postinoculation (dpi). Data are shown as means ± SEM from triplicate wells. (F) Immunofluorescence images showing HEV ORF2-positive cells (green) in polarized F2 cell cultures 5 and 10 days after infection with WT or ORF3null HEV (103 GE per cell) in either the presence or absence of a JAK inhibitor (JAKi) (0.5 μM). Nuclei were counterstained with DAPI. Bars, 100 μm. (G) HEV-positive areas at 5 and 10 dpi were quantified using ImageJ software (NIH). Data are shown as means ± SEM from at least 2 independent experiments, each with duplicate wells. (H and I) Polarized F2 cells were inoculated with WT or ORF3null HEV (103 GE per cell), and the levels of HEV RNA (H) and interferon-stimulated genes (ISGs) (I) were quantified by qRT-PCR at the indicated times. Data are shown as means ± SEM from 2 independent experiments, each with duplicate wells. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.