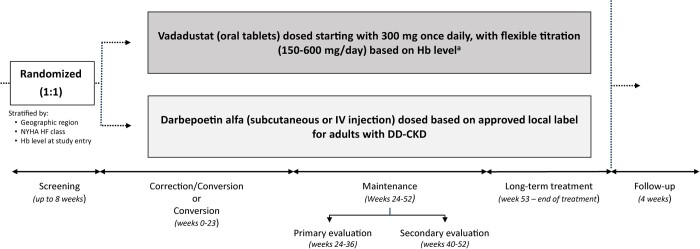
FIGURE 1.
Study design. After a screening period of up to 8 weeks, eligible patients were randomized 1:1 to vadadustat or darbepoetin alfa. Patients in both trials entered four sequential study periods for treatment and evaluation of safety and efficacy: a correction or conversion period (Weeks 0–23), a maintenance period (Weeks 24–52) comprising both a primary (Weeks 24–36) and secondary (Weeks 40–52) efficacy evaluation period, a long-term treatment period (Weeks 53 to end of treatment) and a 4-week safety follow-up period. aStudy drug is titrated to achieve target Hb levels (USA: 10–11 g/dL; non-USA: 10–12 g/dL).