Fig. 2.
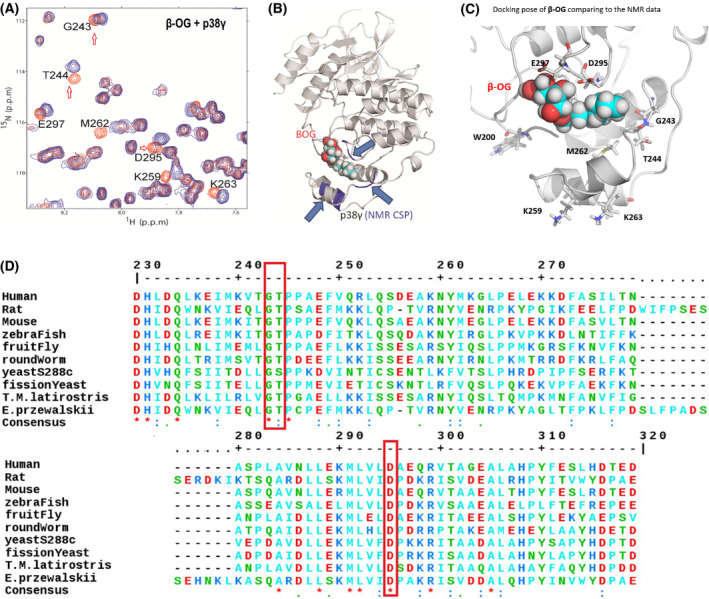
NMR demonstrates a lipid‐binding site on p38γ for β‐OG. (A) 2D NMR CSP experiment locates the p38γ residues that significantly shifted due to addition of β‐OG, indicated in orange. NMR CSPs data by monitoring 1H‐15N HSQC and 1H‐13C HMQC spectra upon the addition of β‐OG alone and p38γ to β‐OG with a molar ratio of 1 : 10. The red hollow arrows indicate three residues, G243, T244, and D295 that are conserved among species from human to yeast (See also Figure 2D, red bracket). (B) The structures of p38γ in complex with β‐OG. A 3D structural model presenting where β‐OG bound to LBD of p38γ, based on NMR CSP titration data. The p38γ crystallography structure coordinate datasets were downloaded from RCSB PDB [PDB id 1cm8 [39]]. (C) β‐OG binds p38 γ in the MKI region of in the lipid‐binding domain and residues which greatly perturbed residues in NMR CSP assay are W200, M262, G243, D295, and E 297. K259 and K263 are consistent with that of alpha p38 which are perturbed by B‐OG in Figure 1F. (D) ClustalW2 consensus alignment diagram of amino acid sequences in the lipid‐binding domain of p38γ among indicated species. β‐OG NMR CSP perturbed residues of human p38γ: G243, T244, and D295 (in red brackets) are conserved among species including yeast. Three residues E297, K259, and M262 share homology with mouse.
