Fig. 7.
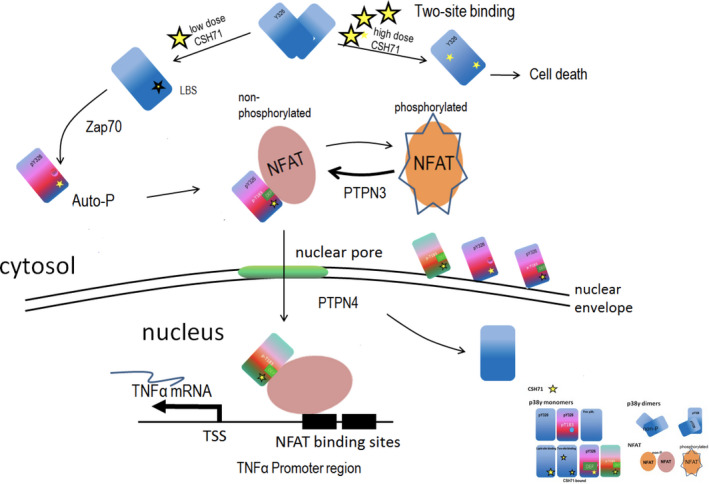
CSH71 as a LBD binder and its biological functionality in CTCL cells. Proposed schematic molecular mechanism by which CSH71 (250 nm) induces TNFα, first to increase cytosolic Y326 phosphorylation of p38γ at the TCR complex proximal to the plasma membrane of T cells and then migrate into the nucleus. Alternative p38 phosphorylation is activated in CTCL cells upon lipid binding, which extends conformational changes to the active loop of p38, followed by interlobe orientation changes, evidenced at Y326 and regions surrounding DEF pocket perturbation, which suggest formation of a DEF site interaction pocket and its availability to other substrates/factors, such as NFATs. Nuclear NFATs then subsequently transcriptionally activate TNFα mRNA in CTCL cells, evidenced by RNA sequencing and pathway analysis at CSH71 treatment (250 nm). At a higher dosage, it is very likely that CSH71 binds to two sites (ATP binding and LBS binding) of p38γ and prevents its dimerization, which further blocks the interaction of p38γ and NFAT thereby preventing its subsequent translocation into the nucleus and thereby cell death.
