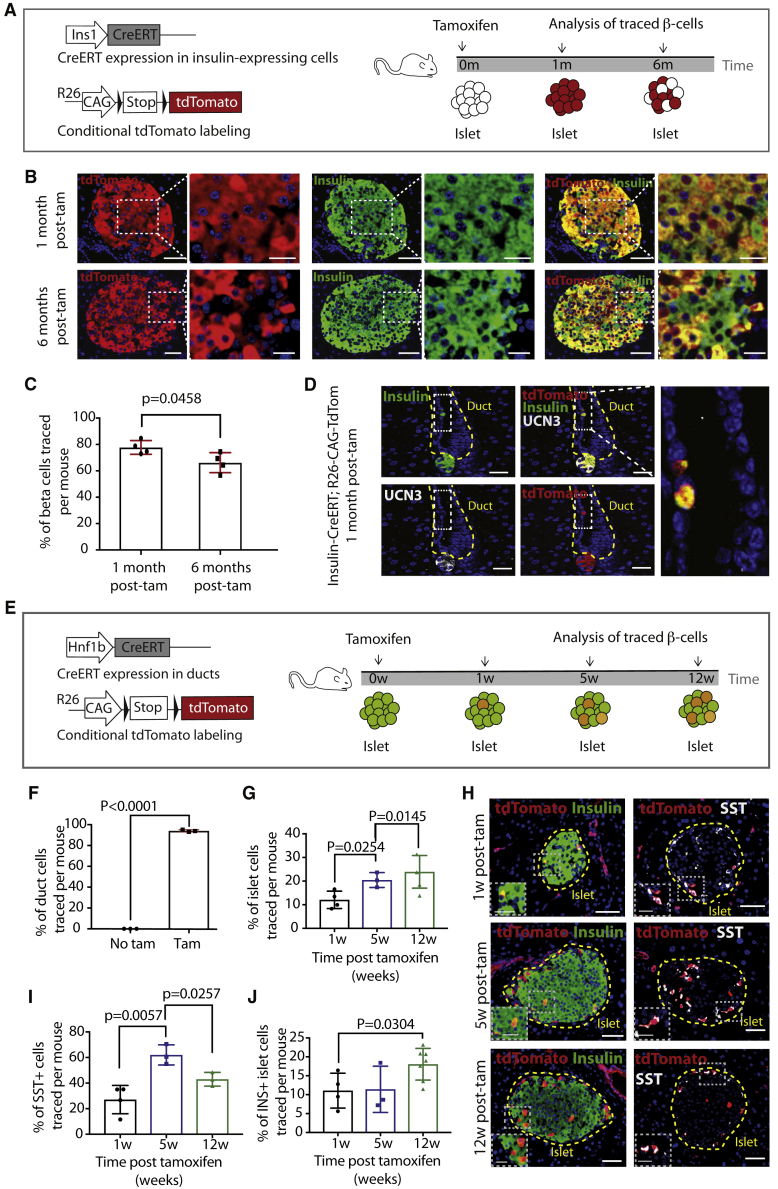
Figure 1.
The β cell population is not solely maintained by existing β cell proliferation
(A) Lineage tracing strategy and experimental timeline.
(B) Double immunofluorescence (IF) for tdTomato and INS in INS-CreERT; R26-CAG-tdTomato mice 1 and 6 months after tamoxifen, with high magnification shown on the right. Scale bars, 50 μm (left) and 20 μm (right).
(C) Quantification of the percentage of INS+ islet cells traced per mouse (N = 4) at both time points (10–26 weeks of age at injection). Data are represented as mean with SD and p value is indicated.
(D) Triple IF for tdTomato, INS and Urocortin3 (UCN3). A traced ductal INS+/UCN3− (boxed) is shown at high magnification. Scale bars, 25 μm (left) and 10 μm (right).
(E) Lineage tracing strategy and experimental workflow.
(F) Quantification of the percentage of ductal cells labeled 1 week after tamoxifen (N = 3 at 8 weeks of age at injection). Data are represented as mean with SD and p values are indicated.
(G) Quantification of the percentage of islet cells traced per mouse. N = 4 at each time point (8–16 weeks of age at injection). Data are represented as mean with SD and p values are indicated.
(H) Double IF for tdTomato/INS and tdTomato/SST. High magnification is shown in the inset (white dashed line). Scale bars, 50 μm.
(I) Quantification of the percentage of SST+ islet cells traced per mouse. N = 4 at each time point (8–16 weeks of age at injection). Data are represented as mean with SD and p values are indicated.
(J) Quantification of the percentage of INS+ islet cells traced per mouse. N = 4 at each time point (8–16 weeks of age at injection). Data are represented as mean with SD and p values are indicated.
See also Figure S1.