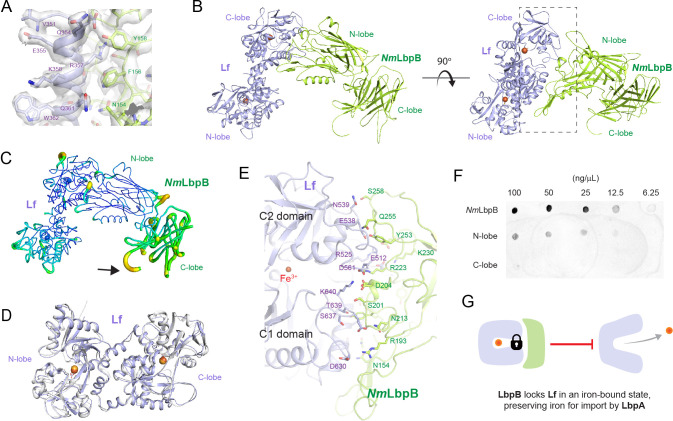
Figure 3. The 2.85 Å crystal structure of N. meningitidis LbpB (NmLbpB) in complex with human lactoferrin.
(A) Zoomed view at the interface between NmLbpB and lactoferrin (Lf) depicting the quality of the electron density shown as a grey isosurface (2FO–FC, 1.0σ). (B) Orthogonal views of the complex with NmLbpB in green, Lf in violet, and the iron atoms as red spheres. The N-lobe of NmLbpB interacts with only the C-lobe of Lf along an extended interface. (C) The C-lobe of NmLbpB has high B-factors with the large loops of this lobe not observed in our structure; the black arrow indicates the putative location of these loops. (D) An alignment of Lf from the complex with the structure of uncomplexed Lf (PDB ID 2BJJ) shows very little conformational changes upon binding NmLbpB (root-mean-square deviation (RMSD) of 1.3 Å). (E) A zoomed view of the binding interface shows extensive interactions along an elongated surface covering both the C1 and C2 domains of Lf (buried surface area 1760.8 Å2). (F) Solid-phase-binding assays show Lf binds both full-length and N-lobe NmLbpB, but not C-lobe only, supporting the observations in the complex structure. (G) Much like what has been proposed for the role of transferrin-binding protein (Tbp) B in the Tbp system, here we propose that lactoferrin-binding protein B (LbpB) also serves to bind and lock Lf in an iron-bound state for delivery to LbpA for iron import.