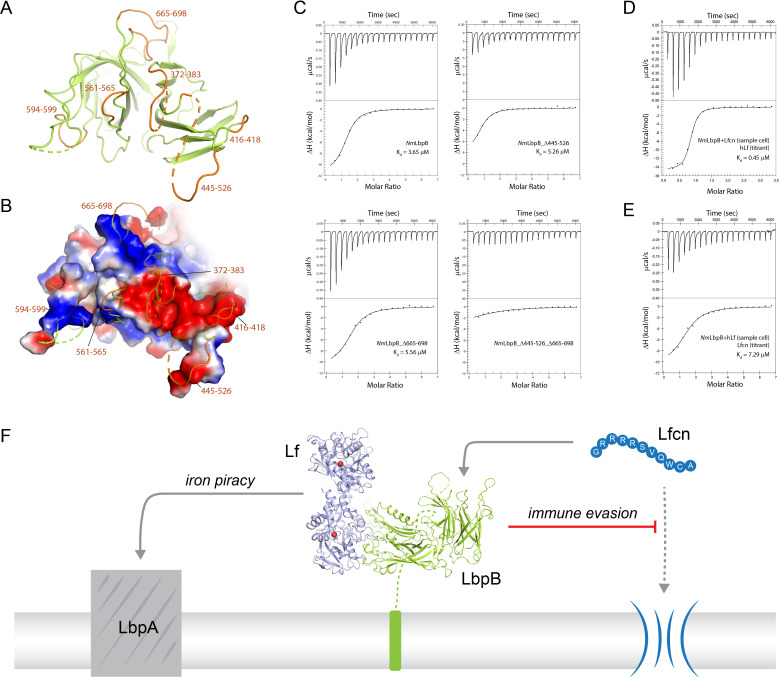
Figure 6. Probing the putative binding interface of N. meningitidis LbpB (NmLbpB) with lactoferricin.
(A) A zoomed view of the C-lobe of NmLbpB with the loops indicated in orange. (B) An electrostatic surface potential representation along the C-lobe of NmLbpB depicting the charged surfaces, including a strongly electronegative region (red). (C) Analysis of the binding parameters using isothermal titration calorimetry (ITC) analysis of wild type and loop-deletion mutants of NmLbpB measuring the effects on lactoferricin (Lfcn) binding. (D) ITC analysis of the NmLbpB–Lfcn complex titrated with lactoferrin (Lf), showing comparable binding to NmLbpB alone. (E) ITC analysis of the NmLbpB–(Lf) complex titrated with Lfcn, showing comparable binding to NmLbpB alone. (F) Model for the dual function of LbpB in mediating Neisserial pathogenesis by serving in both iron piracy and as an antimicrobial peptide (AMP) sink. While not shown, processing by NalP produces a soluble version of LbpB which can diffuse into the host environment to actively locate and neutralize AMP threats.