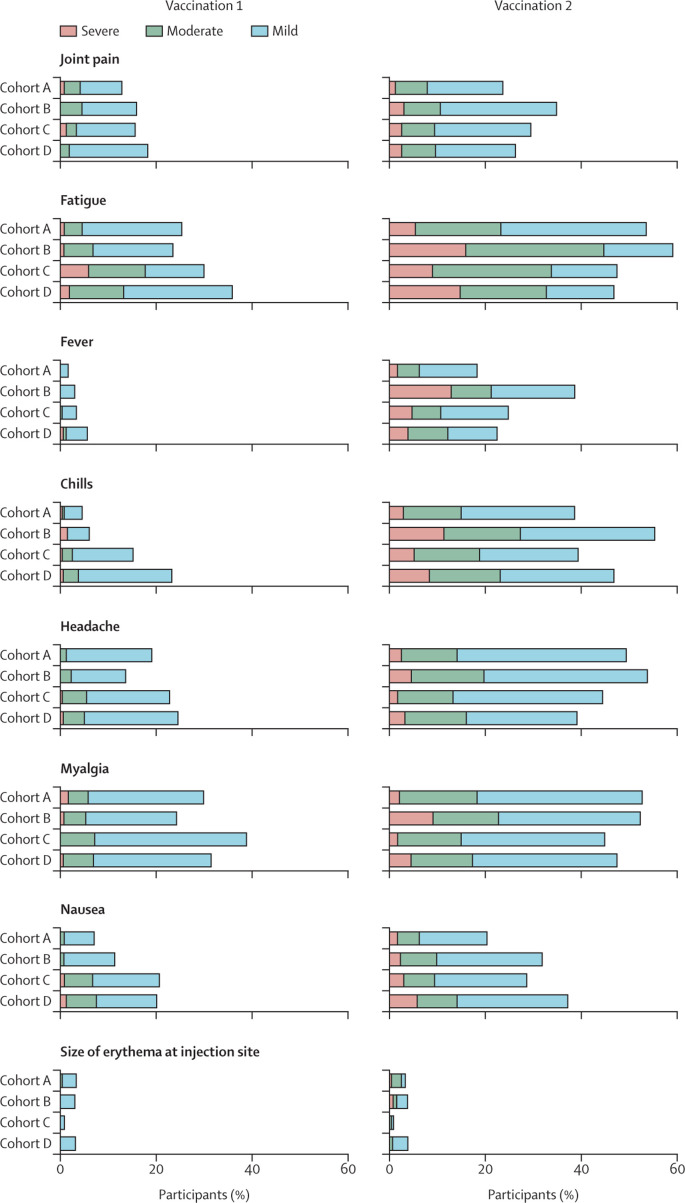
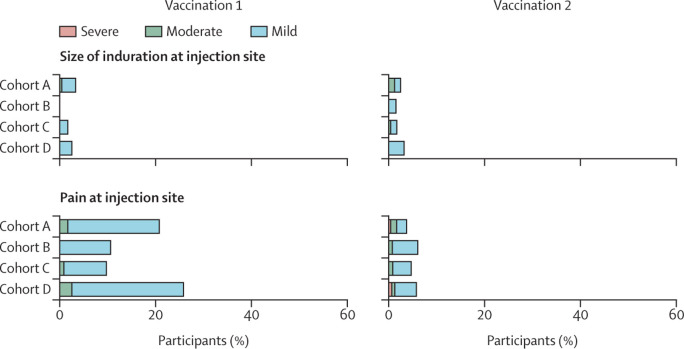
Figure 4.
Solicited adverse events in participants who received at least one vaccination and had no seroconversion at baseline
Participants without cancer were included in cohort A, and patients with solid tumours treated with immunotherapy were included in cohort B, those treated with chemotherapy were included in cohort C, and those treated with chemoimmunotherapy were included in cohort D. Horizontally stacked bar plot showing the percentage of participants reporting solicited systemic and local adverse events up to 7 days after the first and second vaccination. The highest reported grade during the 7 days after each vaccination was included. Solicited systemic and local adverse events that were reported already at baseline were excluded. Mild indicates not interfering with daily activities, moderate indicates interfering with daily activities, and severe indicates could not perform daily activities for joint pain, fatigue, chills, headache, myalgia, nausea, and pain at injection site. For fever, mild indicates a temperature of 38·0–38·4°C, moderate indicates 38·5–38·9°C, and severe indicates 39·0°C or above. For size of erythema and induration at injection site, mild indicates 2·5–5·0 cm, moderate indicates 5·1–10·0 cm, and severe indicates larger than 10·0 cm.