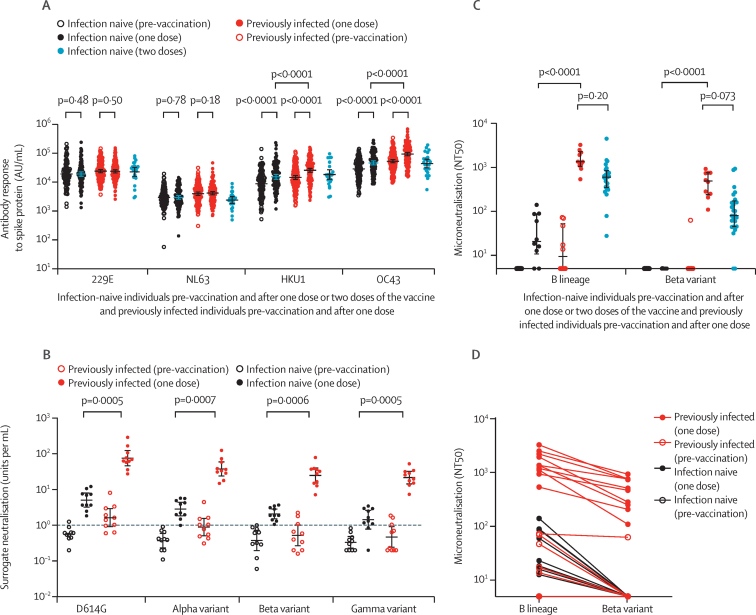
Figure 4.
Antibody responses following BNT162b2 vaccine in naive and previously infected individuals to seasonal human coronaviruses and SARS-CoV-2 variants of concern
(A) Comparison of antibody responses to spike proteins from seasonal human coronaviruses 229E, NL63, HKU1, and OC43 before and after vaccination in 111 infection-naive and 142 previously infected individuals following a single dose and 25 infection-naive individuals following two doses. (B) Surrogate neutralisation activity before and after one vaccine dose in ten naive and ten previously infected individuals to spike proteins from SARS-CoV-2 variants, including variants of concern. Activity is expressed as units per mL with 1 unit per mL equivalent to 1 μg/mL of neutralising activity of the anti-spike monoclonal antibody standard. Thresholds for positivity, based on mean plus 3 SD from 23 pre-pandemic negative control samples, were 1·02 units per mL for D614G, 1·13 units per mL for the alpha (B.1.1.7) variant, 0·93 units per mL for the beta (B.1.351) variant, and 0·98 units per mL for the gamma (P.1) variant. The horizontal dashed line denotes 1 unit per mL. (C) Ability of plasma from ten infection-naive and ten previously infected individuals, before and after a single vaccine dose, to neutralise live virus, expressed as the reciprocal titre required for 50% reduction in infectious focus-forming units (NT50) of lineage B and the beta variant of SARS-CoV-2 in a microneutralisation assay. Microneutralisation data from the 25 naive individuals sampled at 7 days following two doses have been previously described7 and are presented here for comparison. (D) Reduction in neutralisation titres (NT50) for each plasma sample against the beta variant compared to a B lineage virus. Antibody data are presented on a log10 scaled axis for visualisation, with statistical comparisons done on untransformed data. AU=antibody units.