Figure 1.
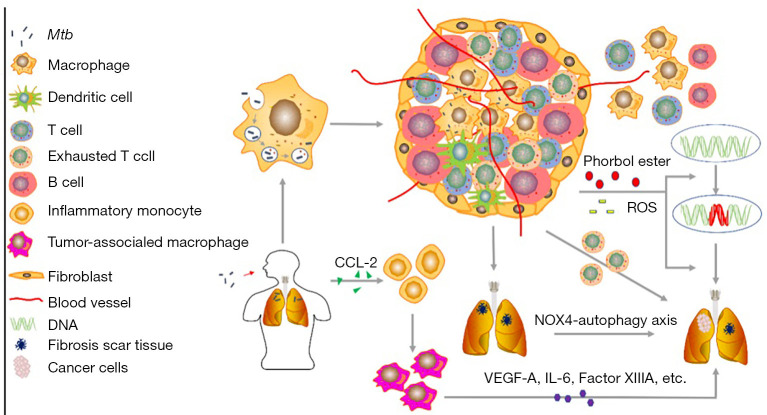
Chronic inflammation elicited by Mycobacterium tuberculosis (MTB) may be involved in the development of lung cancer (LC) through exhausted T cell phenotype, DNA damage and repair, reactive oxygen species (ROS) production, tuberculosis (TB) granuloma, tuberculous fibrosis formation and IMs infiltration. In the early stage of infection, the endocytosed infected MTB escape the phagosome of alveolar macrophages leads to the formation of granuloma, which vascularizes surrounding tissues and recruits immune cells, contributes to the prolonged inflammation. The exhausted T cells enriched in granuloma and the chronic inflammation induced ROS and chemical irritants releasing promote the occurrence of LC. There are a large number of immune checkpoints expression in exhausted T cells, which play a central role in inhibiting the T cell response and facilitating tumor progression. LC can also develop from fibrotic scar induced by MTB-induced lengthy pulmonary inflammation through mechanisms like NOX4-autophagy axis enhances the tumorigenic potential of lung cells. The TB patients have an increased expression level of monocyte chemoattractant protein-1 (CCL-2), which can recruit inflammatory monocytes (IMs). IMs can differentiate into tumor-associated macrophages (TAMs), and IMs-derived TAMs contribute to tumorigenesis through vascular endothelial growth factor A (VEGF-A), IL-6 and epidermal growth factor (EGF) or Factor XIIIA.