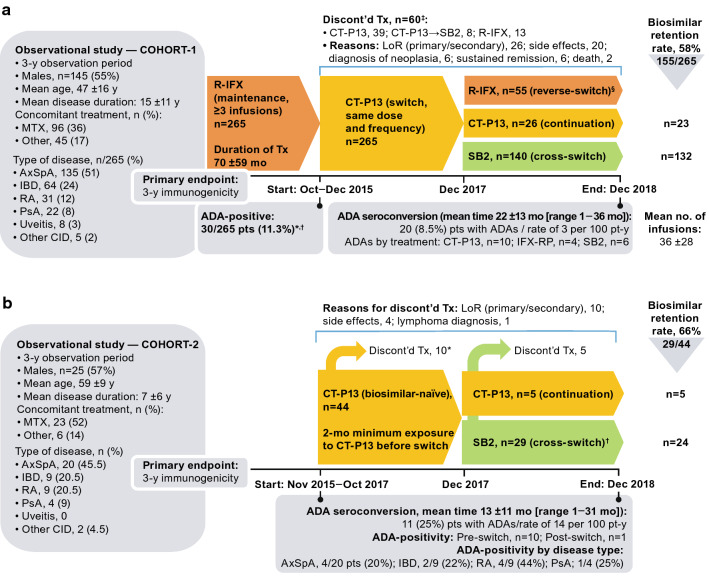
Fig. 7.
Cross-switching immunogenicity study in patients with chronic inflammatory diseases who were on infliximab maintenance (Cohort-1) or who were biologic-naïve (Cohort-2) [1]. a Study flow and results for Cohort-1. *12/30 ADA-positive pts completed the cross-switch; 7/12 gradually became seronegative and 5/12 remained ADA-positive. †5/30 ADA-positive pts continued R-IFX therapy despite undetectable trough and exhibited a good clinical response. ‡20/60 pts (33%) who discontinued treatment were ADA-positive versus 30/205 (15%) pts who reverse-switched (p = 0.002). §Pts (n) who reverse-switched did so after [no. of CT-P13 infusions]: 19 pts [1], 14 [2], 17 [3], and 5 [> 3]. b Study flow and results for Cohort-2. *Among the patients who discontinued treatment because of LoR (primary or secondary), 4 were ADA-positive and 2 halted treatment due to an infusion-related reaction (both ADA-positive). †Cross-switch groups consisted of AxSpA, n = 11 pts; IBD, n = 8; RA, n = 7; PsA, n = 1; uveitis, 0; and other CID, n = 2. ADA antidrug antibody, AE adverse event, AxSpA axial spondyloarthritis, CD Crohn’s disease, CI confidence interval, CID chronic inflammatory disease, CRP C-reactive protein, CT-P13 biosimilar infliximab, discont’d discontinued, IBD inflammatory bowel disease, LoR loss of response, mo months, MTX methotrexate, PsA psoriatic arthritis, RA rheumatoid arthritis, R-IFX infliximab reference product, SB2 biosimilar infliximab, Tx treatment, UC ulcerative colitis, y year