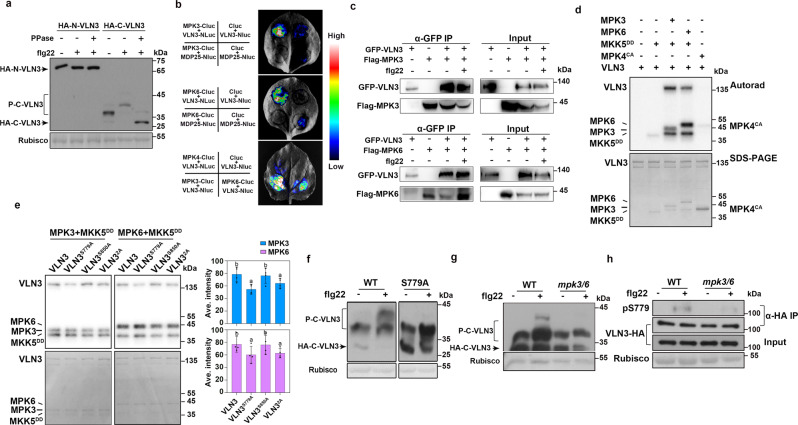
Fig. 2. VLN3 is phosphorylated by MPK3/6 during plant innate immunity.
a The C terminus of VLN3 is phosphorylated upon flg22 activation. HA-tagged N-VLN3 and C-VLN3 were expressed in Arabidopsis protoplasts. Following treatment with mock or 100 nM flg22 for 10 min, total protein was separated in a phos-tag gel and VLN3 fragments were detected using anti-HA antibodies. Rubisco was used as a loading control. b-c MPK3/MPK6 interact with VLN3 in vivo. The indicated constructs were transiently expressed in N. benthamiana. Luciferase complementation imaging assay (b) and coIP assay (c) was performed. Nluc N-terminal fragment of firefly luciferase, Cluc C-terminal fragment of firefly luciferase. d Activated MPK3 and MPK6 phosphorylate VLN3 in vitro. VLN3 proteins were incubated with MPK3/6 activated by MKK5DD or a constitutively active form of MPK4 (MPK4CA)50 in an in vitro kinase assay. VLN3 phosphorylation was detected by autoradiography after gel electrophoresis. e–f Ser779 is required for VLN3 phosphorylation in vitro (e) and in vivo (f). e Phosphorylation of wild-type and mutated VLN3 was assessed using an in vitro kinase assay. VLN3 phosphorylation was detected by autoradiography after gel electrophoresis and the band intensity was quantified by densitometric analyses. f Protoplast expressing C-VLN3 and C-VLN3S779A tagged with HA were treated with flg22. Total protein was subject to phos-tag gel analyses. g MPK3/MPK6 are required for VLN3 phosphorylation in vivo. The HA-C-VLN3 was expressed in MPK6SR protoplasts. Prior to 10-min flg22 (100 nM) treatment, the MPK6SR protoplasts were treated with mock or NAPP1 (2.5 µM) for 12 h. The phosphorylation of C-VLN3 was detected by phos-tag gel analyses. h Flg22 induces Ser779 phosphorylation in a MPK3/6-dependent manner. HA-VLN3 was expressed in protoplasts of MPK6SR rosette leaves. The MPK6SR protoplasts were treated with mock or NAPP1 (2.5 µM) for 12 h before treatment with 100 nM flg22 for 10 min. HA-VLN3 protein was affinity purified with anti-HA antibodies, and Ser779 phosphorylation was detected by immunoblotting with anti-pSer779 antibodies. Total HA-VLN3 protein was detected by anti-HA immunoblot. Values in (e) are means ± SD. n = 4. Different letters indicate significant differences at P < 0.05, as determined by two-way ANOVA with Tukey’s multiple comparisons test. The exact p values are provided in the Source Data file. The experiments in (a, c, d, f–h) were repeated three times with similar results.