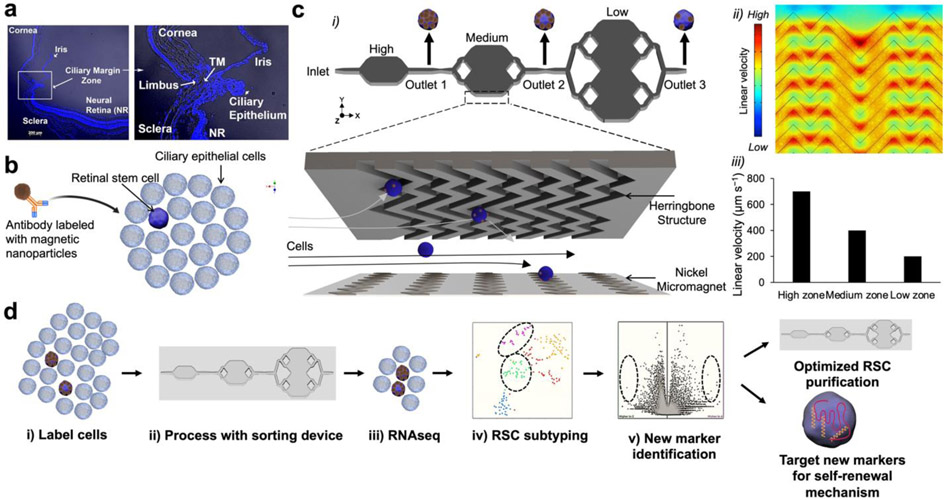
Figure 1.
RSC purification approach & magnetic resolution fluidic device design. (a) A mouse eye is enucleated, and primary cells are dissected from the ciliary epithelium (CE) of the mouse eye. (b) The CE is dissociated and incubated with antibodies specific to RSC cell-surface proteins. The antibodies are labeled with magnetic nanoparticles (MNPs) via biotin-streptavidin coupling. (c) i. The cells tagged with MNPs are loaded into a three-zone microfluidic device featuring surface ridges and nickel micromagnets. Cells with high magnetic loading are captured in the high capture zone, whereas cells with medium to low magnetic loading are captured in medium and low capture zones, respectively. (c) ii. Simulation of fluid flow within the device. High linear velocity regions generated by the grooved surface (red circle) bring the MNPs tagged cells in contact with the nickel micromagnets where they become captured. (c) iii. Average linear velocities calculated for each capture zone within the cell capture device. (d) Experimental workflow: The cells are first labeled with antibodies, sorted in the device to enrich the cells. The individual enriched cells are then subjected to single-cell RNA sequencing which allows for the identification of two clusters that contained RSCs with differentially expressed cell-surface markers and intracellular markers. The novel cell-surface markers were then used to further purify the RSCs from the CE from 1:500 to 1:2. To test if any of the intracellular marker-encoding genes and transcription factors have any functional roles in RSCs, they were assayed either in a mouse model or using siRNA knockdown in the clonal stem cell sphere assays.