Abstract
Background
Laboratory monitoring of mother, fetus, and newborn in hemolytic disease of fetus and newborn (HDFN) aims to guide clinicians and the immunized women to focus on the most serious problems of alloimmunization and thus minimize the consequences of HDFN in general and of anti-D in particular. Here, we present the current approach of laboratory screening and testing for prevention and monitoring of HDFN at the Copenhagen University Hospital in Denmark.
Summary
All pregnant women are typed and screened in the 1st trimester. This serves to identify the RhD-negative pregnant women who at gestational age (GA) of 25 weeks are offered a second screen test and a non-invasive fetal RhD prediction. At GA 29 weeks, and again after delivery, non-immunized RhD-negative women carrying an RhD-positive fetus are offered Rh immunoglobulin. If the 1st trimester screen reveals an alloantibody, antenatal investigation is initiated. This also includes RhD-positive women with alloantibodies. Specificity and titer are determined, the fetal phenotype is predicted by non-invasive genotyping based on cell-free DNA (RhD, K, Rhc, RhC, RhE, ABO), and serial monitoring of titer commences. Based on titers and specificity, monitoring with serial peak systolic velocity measurements in the fetal middle cerebral artery to detect anemia will take place. Intrauterine transfusion is given when fetal anemia is suspected. Monitoring of the newborn by titer and survival of fetal red blood cells by flow cytometry will help predict the length of the recovery of the newborn.
Keywords: Hemolytic disease of fetus and newborn; Alloimmunization; Cell-free DNA; Middle cerebral artery, Peak systolic velocity; Next-generation sequencing
Introduction
Alloimmunization is the process where an individual lacking a specific antigen of a blood group is exposed to the antigen and responds by producing specific antibodies. Exposure might occur by transfusion with donor blood, or by accidental transfer of fetal red blood cells (RBCs) to the pregnant woman, such as fetomaternal hemorrhage [1, 2, 3].
Active transplacental transfer of maternal antibodies via the neonatal Fc receptor [4] will take place when the antibody production has switched from the initial IgM response to IgG. Transfer will accelerate in the 2nd and 3rd trimester and can lead to hemolytic disease of fetus and newborn (HDFN) [5].
The essential clinical manifestation of HDFN is fetal and neonatal anemia. This is observed as erythroblastosis fetalis, hepatic dysfunction leading to hypoalbuminemia, ascites, hydrops fetalis, congestive heart failure, intrauterine growth retardation, abdominal and pericardial edema, antenatal asphyxia, acute bilirubin encephalopathy, and kernicterus spectrum disorder [6]. The concentration of unconjugated bilirubin might surpass albumin bilirubin binding capacity and translocate across the brain-blood barrier with subsequent accumulation in basal ganglia resulting in neuronal-cell death. Bilirubin encephalopathy, or kernicterus, may lead to minor neurodevelopmental disabilities, nerve deafness, spastic cerebral palsy, or even death [7].
Laboratory monitoring is an important tool to predict a potential risk; but it cannot with certainty forecast clinical severity for the fetus. However, crucially, the laboratory setup removes the urgency of the diagnosis of HDFN and allows timely implementation of tested diagnostic and therapeutic measures. Cases of unpredicted HDFN still occur and they are consequently not included in the antenatal fetal screening program [2, 3, 8].
It is important at an early gestational age (GA) to determine if the woman is RhD negative and thus is at risk of producing the most frequent alloantibody, anti-D, and whether or not she is amenable to preventive treatment, Rh prophylaxis, later in pregnancy. At the same time all women are screened for the presence of any alloantibody.
In Denmark, if the woman is typed RhD negative and has no alloantibodies in the 1st trimester antibody screen, she will be offered a non-invasive prediction of the fetal RhD blood group at GA 25 weeks at routine consultation with her general practitioner. At the same time, an antibody screen test is performed. If the fetus is RhD positive and the women is non-immunized, anti-D immunoglobulin (RhIg) is offered at GA 29 weeks and again after delivery. This is described below (see Antenatal RHD Screening).
In contrast, if a potentially harmful maternal antibody is detected in the screen in the 1st trimester, it is essential to determine if the present fetus carries the allele for the antigen targeted by the maternal antibody. Only in this case is the fetus at risk of developing HDFN. In cases where the fetus is at risk, intensified pregnancy monitoring and treatment by transfusion can be instituted, and in cases where risk of HDFN due to known alloantibodies can be excluded, a less intensive and financially less burdensome approach can be taken. Also, much anxiety from the prospective parents can be avoided. If the woman is alloimmunized to the RhD antigen when tested in the 1st trimester in the antibody screen, we immediately use the same antenatal RHD screening assay as we use for non-immunized women in GA 25 weeks. Detection of the RHD gene is based on selective amplification of fetal DNA encoding the RHD gene.
Selective amplification is, however, not reliably achievable for other blood group polymorphisms [9]. We examine women alloimmunized to the other prevalent antigens (K, RhC, Rhc, RhE, and ABO) by non-invasive antenatal molecular diagnostics that amplify single nucleotide variants (SNVs) potentially present in the cell-free DNA (cfDNA), maternal as well as fetal. We describe our clinically implemented non-invasive methods based on cfDNA for 1st/2nd trimester determination of the genes encoding the clinically most important targets of alloantibodies (see Non-Invasive Prediction of Fetal K, RhC, Rhc, RhE, and ABO Blood Group).
Prediction of other phenotypes based on antenatal genotyping has not yet been implemented in our laboratory. Instead, we do a paternal phenotype if antibodies to the relevant antigens are available and make a statistical risk assessment based on that. Blood group antibodies anti-A and anti-B are responsible for neonatal hemolysis and hyperbilirubinemia, which in rare cases necessitate treatment with transfusion (see Maternal ABO Antibodies).
Flow cytometry (FC) is a useful method for small population detection and quantification, for example, after intrauterine transfusion (IUT) for detection of fetal RBCs and donor RBCs. Also, minute samples of fetal blood can be examined for multiple parameters improving laboratory guidance (see FC in HDFN, Fetus and Newborn). In this paper, we present the procedures related to laboratory screening and monitoring in HDFN as currently performed at the Copenhagen University Hospital, Rigshospitalet, in Denmark.
Routine Blood Group Typing and Screening for Irregular Antibodies, Rh Prophylaxis
Blood samples from the 1st trimester initial pregnancy consultation with the general practitioner are typed for ABO and RhD blood groups and an antibody screen is performed. We use automated equipment and Capture-R® Ready-Screen (I and II) for detection of IgG antibodies to RBC antigens. Typing identifies the RhD-negative women who can develop anti-D antibodies. Antibody screening identifies those who have already developed alloantibodies in the 1st trimester, whether RhD positive or RhD negative.
At GA 25 weeks, RhD-negative women with a negative 1st trimester antibody screen are offered routine non-invasive fetal antenatal RHD screening, and the repeated routine antibody screening is offered only to RhD-negative women (see Antenatal RHD Screening). At GA 29 weeks, the nonimmunized pregnant woman is offered intramuscular injection of 250–300 μg RhIg by the midwife if RHD is detected in the cell-free fetal DNA (cffDNA) from plasma. The 250–300 μg RhIg injection is repeated within 72 h after delivery. Investigation for fetomaternal hemorrhage and quantification by flowcytometry is only made if hemorrhage is suspected.
Investigating and Monitoring Irregular Antibodies
For alloimmunized women, identification of the target antigen of the alloantibody will be conducted to provide further information on the potential clinical impact of the alloimmunization. Maternal antibodies targeting a distinct blood group antigen often lead to a known distinct pattern of clinical manifestations. This knowledge guides the planning of the laboratory monitoring and the fetal specialist surveillance.
For the antibody identification we use 11 different reagent in-house single donation glycerol frozen-thawed RBCs and anti-IgG column agglutination technique (CAT). The woman's own RBCs are included in the panel to distinguish allo- and autoantibodies. If Rh antibodies are suspected the examination is extended with a panel of papain-treated RBCs. Per definition, for an alloantibody to be present, a phenotype of the woman's own RBCs should demonstrate absence of the target antigen of the alloantibodies.
Some antibodies have empirically been found to be of no clinical significance (anti-N, -Lea, -Leb, -A1, -IH, -I), whereas others are known to be of potential dire consequences (anti-K, -c) and referral to a fetal medicine center should be considered regardless of titer, and yet another group (anti-D, -C, -E, -e, -Cw, -Kpa, -Kpb, -k, -Jka, -Jkb, -Fya, -Fyb, -S, -s, -Wra, -M, -P1, -Lua, and -Lub) is referred to a fetal medicine center if a titer above 16 is measured.
At GA 25 and 32 weeks, the alloimmunized woman is routinely examined with a measurement of titer and with a screening for additional antibodies. A titer above 16 for the latter group of antibodies is empirically determined as the threshold value indicating increased risk of HDFN and warrants closer surveillance by a fetal medicine specialist with serial Doppler ultrasound measurements of the peak systolic velocity (PSV) in the fetal middle cerebral artery (MCA) [10, 11].
Semi-quantification of alloantibody is done by a serial 2-step dilution of plasma in saline followed by examination with CAT. The titer is defined as the inverse of the highest dilution still demonstrating a positive reaction of at least a weak reaction (w+). Determination of titer has an intra-laboratory variation of ±1 titer step and a CV% of 12.4 calculated based on 100 manually titrations conducted in the routine laboratory during 6 months by the routine staff. The material used for this validation was the Working Standard Anti-D for assuring operator and test performance (The National Institute for Biological Standards and Control [NIBSC], Potters Bar, UK; Code No. 07/304). The reagent RBCs we use to determine titer are heterozygous for the target antigen, and the same reagent in-house single donation is used throughout pregnancy to reduce variation. An increase of two dilution steps or more is considered a significant development that deserves special attention by the clinician and possibly referral to the fetal medicine center. Any two-step or more deviation is investigated in the laboratory by comparison with a side-by-side analysis of previous samples. The Working Standard Anti-D is included every day as a control.
An antibody screen represents a “snapshot status” of the antibody content of the pregnant woman at the time of sampling; the titer might increase rapidly because of continuing exposure to fetal or donor RBCs. Within days additional antibodies may also develop. Therefore, a serial monitoring is important.
Antenatal RHD Screening
As part of a targeted RhIg prophylaxis program for non-immunized RhD-negative women, knowledge of the fetal RhD type helps restrict prophylaxis to those women only who carry an RhD-positive fetus [12, 13]. This restriction avoids superfluous exposure to prophylaxis in women carrying an RhD-negative fetus and reduces the overall use of RhIg, which is a limited resource [14, 15].
The fetal RhD status is predicted by analysis of cffDNA in the maternal plasma that also contains maternally derived cell-free DNA. Presence of the fetal RHD gene indicates that the fetus is RhD positive. Since the first reports of cell-free fetal RHD in maternal plasma [16, 17], non-invasive fetal RHD genotyping has become highly integrated into clinical medicine, and its accurate performance has been covered comprehensively in the literature [12, 13, 18, 19, 20, 21, 22, 23, 24].
As an antenatal screening to guide RhIg prophylaxis, non-invasive prenatal testing of fetal RHD has been introduced as a nationwide clinical service in several European countries [16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. Evaluations of national programs have demonstrated high test accuracy, with sensitivities of >99.9% around 25 weeks of gestation and >99% from GA 10 weeks [13]. Recent recommendations for validation and quality assurance of fetal RHD genotyping have been prepared [25].
The Copenhagen setup for antenatal RHD screening has been described in detail [26, 27, 28]. Briefly, blood samples are taken by the general practitioner at GA 25 weeks. Blood samples arrive at the laboratory after an average of 4 days in transport (up to 7 days are accepted). Plasma is separated and DNA is extracted from 1 mL of plasma. Eluted DNA is tested by real-time PCR targeting RHD exons 7 and 10 in a duplex manner with the same dye, which increases the analytical sensitivity [29]. The RHD PCR is sensitive enough to detect one genome equivalent (geq) per PCR [30], and the overall detection limit of the setup is 6 geq per mL. Total DNA is targeted by GAPDH as quality control for sample handling and DNA purification.
Based on the amplification of fetal RHD exon targets, the sample is predicted to be positive or negative, or inconclusive. If positive or inconclusive, the pregnant woman is recommended to receive prophylaxis. Some maternal RHD variants may give a positive result masking the detection of the fetal RHD. In such cases, the result is determined to be inconclusive, and prophylaxis is offered to the woman. As an outcome of implementing nationwide non-invasive prenatal testing for fetal RHD, unnecessary antenatal prophylaxis is avoided in 97.3–99.6% of the women carrying an RhD-negative fetus [28, 31, 32]. Future applications may include expanding the targeted approach to RhD-negative pregnant women with early sensitizing events [33].
Non-Invasive Prediction of Fetal K, RhC, Rhc, RhE and ABO Blood Group
We have recently reported a procedure based on next-generation sequencing (NGS) analysis of PCR-amplified cfDNA from maternal plasma for prediction of the fetal blood group [34, 35, 36, 37]. As some fetuses may die from HDFN as early as GA 18 weeks, it is necessary to be able to predict the fetal blood group early in pregnancy. We use this general approach to predict fetal K, RhC, Rhc, RhE, and ABO blood groups in cases with a risk of HDFN due to maternal production of the corresponding antibodies [34, 35, 36, 37].
The NGS based analysis can detect the presence or absence of alleles encoding incompatible antigens on the fetal RBCs. NGS is a powerful technology that enables the parallel sequencing of many million DNA sequences. We use this technology in a very simple approach: cfDNA is purified from 4 mL of maternal plasma and after PCR amplification of the genetic basis of the blood group, the PCR product is sequenced to great depth. The number of times that the blood group SNV in question occurs is counted and the relative frequency of the SNV, exceeding the background threshold, will be the basis of the prediction. As there are some background reads due to errors of PCR amplification and sequencing, this threshold is important to determine empirically. The background is remarkably low with an empirical threshold for a positive sample of approximately 0.05% positive reads.
Preanalytical conditions are important to address as these NGS-based assays rely on maintaining the in vivo ratio of fetal versus maternal SNVs. Thus, it is important to ensure that maternal cells do not contribute DNA after blood sampling. Taking blood samples in Streck tubes is therefore highly recommended. Some factors are important, such as keeping the amplicons short and keeping spurious amplification to a minimum. The volume of plasma interrogated is approximately 1 mL. Finally, the data analysis we currently employ is two-pronged: one analysis of the fastq sequences is done using FastQC software and another analysis is performed using simple string searches with grep in a Linux formatted PC. We do not perform alignment-based analysis.
Even though the prediction of the different blood groups: K, RhC, Rhc, RhE, and ABO is based on the same generic method, there are important differences in respect to primer design and data analysis. For instance, in the case of Rhc prediction, background reads from highly homologous sequences of RHD may complicate the prediction. The ABO prediction requires the combined results of two primer sets for an antigen prediction.
After implementation of the two-pronged data analysis, we have not yet had any discordant results from a small cohort of samples. As a postnatal blood group was not determined in many cases, a significant number of samples have not been used for formally validating the results of the prenatal fetal blood group prediction. Development of laboratory methods, validation as well as continuous quality control, is dependent on meticulous and continuous contribution from laboratories and clinics. O'Brien et al. [38] have used digital PCR for fetal blood group prediction, and Orzińska et al. [39] have also used NGS for fetal blood group prediction.
Maternal ABO Antibodies
ABO incompatibility is now the most prevalent cause of HDFN with hyperbilirubinemia in developed countries due to the success of Rh prophylaxis [2, 6, 40]. A recent Danish study found ABO incompatibility in 15 of 21 cases with total serum bilirubin ≥600 μmol/L, comprising a significant risk of kernicterus spectrum disorder [41]. Furthermore, rare cases of fetal hemolysis, anemia, and hydrops fetalis caused by ABO antibodies have been described [42, 43].
Currently, we do not have a systematic screening procedure for maternal ABO antibodies harmful to the fetus and newborn [44, 45, 46]. Maternal anti-A and anti-B IgG titers are predictive of neonatal requirement for treatment of hyperbilirubinemia [47, 48]. However, we found the positive predictive values both in the 1st trimester (65%) and perinatally (73–76%) to be too low to be used clinically for routine screening and we aim for enhancement of predictive values [49] by on-going research.
We have described the use of two antibody screening methods: (i) solid phase red cell adherence assay (SPRCA) only detecting IgG anti-A and anti-B and (ii) manual anti-IgG CAT detecting both IgG and IgM reacting at 37°C. The two methods yielded comparable results. SPRCA is most suitable for batch analysis, whereas CAT is amenable for single sample analysis.
Standard infant transfusion practice in our health care region is ABO-identical RBCs. Therefore, in addition to an antibody screening test for irregular antibodies, we also perform a determination of regular anti-ABO antibodies of the IgG class of the incompatible newborn to be transfused. Detection of IgG anti-A and anti-B is followed by determination of the maternal IgG anti-A and anti-B titer. This is likely to lead to identification of more women with high-titer IgG anti-A and anti-B.
Laboratory Monitoring, anti-A and anti-B
In pregnancies with identified maternal high-titer IgG anti-A and anti-B or a history of a previous pregnancy where maternal anti-A/B was responsible for HDFN, the anti-A and anti-B IgG titer is determined in the 1st trimester as well as at GA 32 weeks. For the methods described a common cut-off value of 512 was initially found for anti-A/B [49]. However, additional studies (in preparation) showed that distinct cut-off values for anti-A and anti-B increased accuracy. Therefore, we now apply a cut-off value of 512 for maternal anti-A and 256 for anti-B. The cut-off value is used for recommendation of antenatal non-invasive fetal ABO blood group prediction and for fetal monitoring by ultrasound of MCA-PSV in case of incompatible antigens on fetal RBCs. The flow chart presented in Figure 1 presents the complete laboratory monitoring of HDFN.
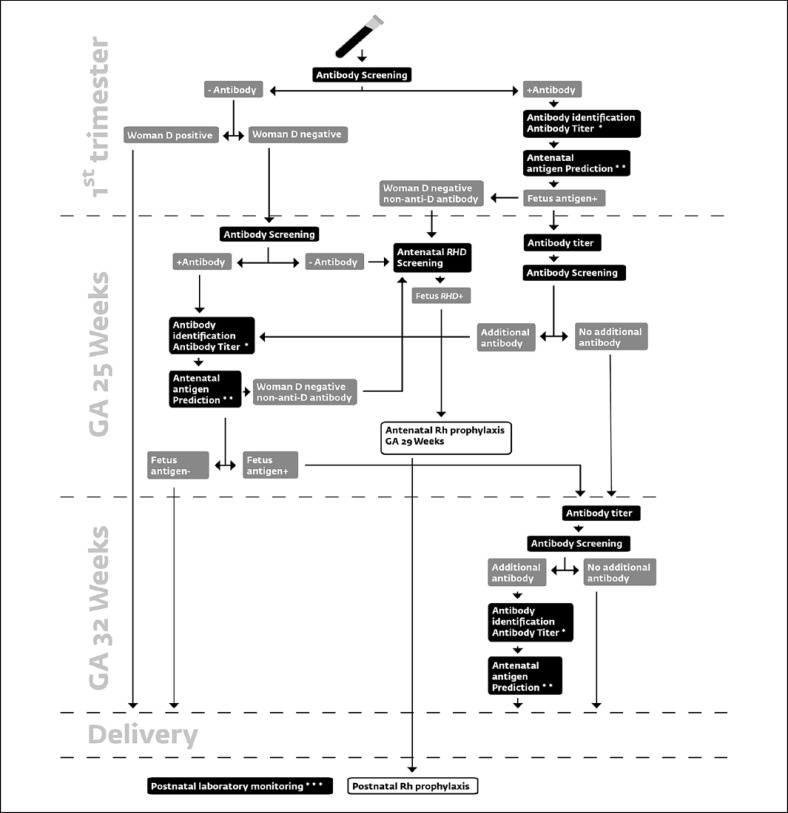
Fig. 1.
The flow chart illustrates the use of individual components of the laboratory HDFN monitoring of all pregnant women. RhD-positive and RhD-negative women are screened for irregular antibodies against RBC antigens in the 1st trimester. Antibodies to ABO blood group antigens are only included in the examination if supplementary information gives an indication to do so. RhD-positive women who test negative at this first antibody screening are not examined later. All RhD-negative women are re-tested for antibodies at GA 25 weeks, and the antenatal RHD screening based on cffDNA is performed. RHD screening showing an RHD-positive fetus leads to the administration of Rh prophylaxis at GA 29 weeks and subsequently also the postnatal prophylaxis. Immunized RhD-negative women are screened for antibodies again at GA 32 weeks. At GA 25 weeks and GA 32 weeks, RhD-positive women with irregular antibodies detected in the first trimester are screened. Alternative timing of examination for both RhD-negative and positive women is followed if the clinician decides so. * Shows a potential trigger for conducting antenatal antigen prediction. The specific criteria for antenatal antigen prediction are: C, c, K, or D at titer ≥1; E at titer >1; A at titer ≥512; B at titer ≥256. For blood groups A and B, also HDFN due to anti-A or anti-B in a previous pregnancy gives an indication for antigen prediction. ** Shows a potential trigger for referral to the fetal medicine center. The specific criteria for referral are: anti-D, -C, -E, -e, -Cw, -Kpa, -Kpb,- k, -Jka, -Jkb, -Fya, -Fyb,-S, -s, -Wra, -M, -P1, -Lua, -Lub titer >16, and anti-K, -c titer ≥1. *** Designates postnatal monitoring of the newborn with titer, serological antigen detection, and flow cytometric quantification of fetal and donor RBCs. A black box designates an analysis, a grey box designates a result, and a white box designates Rh prophylaxis.
FC in HDFN, Fetus and Newborn
Agglutination techniques are informative in most cases, but by supplementing with FC more detailed and semiquantitative information [50] can be produced also in unexpected urgent cases of suspected HDFN where diagnosis is initially uncertain. Determination of fetal and newborn antigens and direct antiglobulin test (DAT)-positive RBCs can be made impossible or inconclusive by access to a limited volume of sample, small surviving populations of fetal cells after multiple IUTs, and due to weak fetal expression of antigens [51]. FC enables quantification of subpopulations, for example several populations of distinct RBC phenotype in cases of mixed populations of donor and patient cells, enabling measurement of the survival of the infant's own RBCs, as well as donor RBCs.
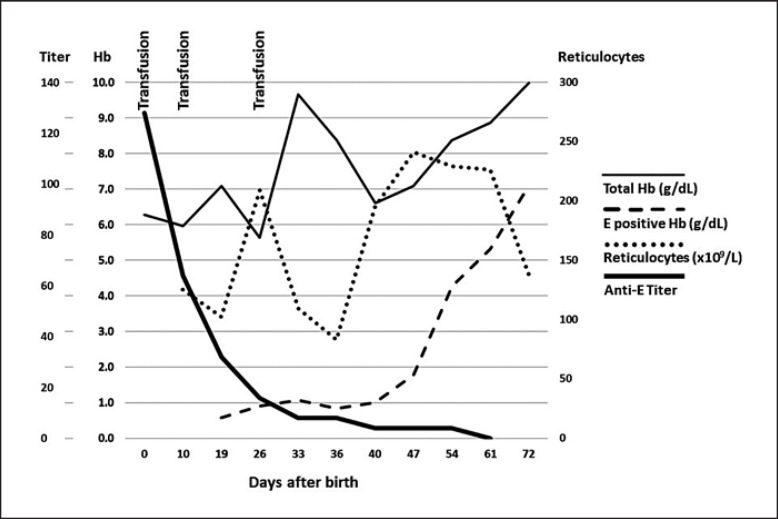
In Figure 2, we present an example of serial monitoring of various parameters of a severely anemic newborn, with hemoglobin (Hb) at birth of 6.3 g/dL (3.9 mmol/L). The RhD-positive woman unexpectedly delivered an anemic infant in GA 38 weeks. Upon investigation after delivery, the mother had an allo-anti-E, titer of 2,048. The anti-E developed between the 1st trimester antibody screening and delivery. The newborn was DAT positive.
Fig. 2.
Serial determinations of parameters for the first 72 days after the birth of the newborn. On day 19, less than 0.6 g/dL (0.4 mmol/L) of the total Hb 7.1 g/dL (4.3 mmol/L) was from endogenous E-positive RBCs. Only after 47 days, at a titer of anti-E below 4, did the E-positive RBCs survive and total Hb started to increase in parallel. Reticulocytes transiently decreased as a response to transfusions, and decreased again after day 61, when E-positive RBCs were being normalized. The newborn parameters were: anti-E titer determined by 2-step dilutions and analysis in CAT; total Hb (g/dL) from donor RBCs and newborn RBCs measured in a hematology analyzer; E-positive Hb was calculated as the E-positive RBC fraction of total RBCs measured by FC multiplied with the total Hb; reticulocytes were measured in a hematology analyzer. Informed consent was obtained from the parents of the infant. The study followed the guidelines of the institutional review board of Copenhagen University Hospital.
Immediately after birth the newborn was given a transfusion with compatible donor RBCs, and again on day 10 and day 26 in accordance with guidelines for treatment of anemia in the newborn. Initially, a hepatic cause, Alagille syndrome, was suspected. However, only HDFN was found. To substantiate the diagnosis of HDFN, we used FC to determine a series of percentages of newborn E-positive RBC.
Newborn E-positive RBCs were identified in FC by reacting RBCs with reagent anti-E followed by anti-human IgG conjugated to a fluorophore, as previously described [52]. Total hemoglobin and reticulocytes were measured with a hematology analyzer. To corroborate E-positive results, we supplemented with measurement by FC of fetal hemoglobin (HbF) and obtained similar results (data not shown) [50].
We observed a close correlation between the waning of the allo-anti-E and an increasing survival of fetal RBCs. Figure 2 demonstrates that the effects of the anti-E is reflected in the newborn RBCs for more than 72 days and that survival of the newborn is dependent on transfusion therapy during the first 47 days.
Generally, if a fetus has received intrauterine transfusion it is possible to monitor the percentage of fetal versus donor RBCs. This can be done in several ways depending on the specific situation, but typically we use as a marker the antigen targeted by the maternal antibodies, positive RBCs are fetal, antigen-negative RBCs are from the donor due to the use of compatible blood. The percentage of fetal RBCs is also measurable with the marker HbF [50].
Determining the Actual Clinical Course, Doppler Ultrasonography MCA-PSV for Non-Invasive Prediction of Fetal Anemia
Maternal alloantibodies and fetal expression of the corresponding RBC antigen is the prerequisite for HDFN. However, a large variation in clinical impact is observed with identical laboratory findings. Even in the same woman clinical variation occurs from one antigen-positive fetus to another despite an unchanged alloantibody titer [53]. Supplementary modalities of monitoring are needed to determine the actual clinical consequence of the alloimmunization.
Measurement of MCA-PSV is the golden standard for non-invasive prediction of fetal anemia. Mari et al. [11] showed that a cut-off of 1.5 multiples of median on Doppler ultrasound measurement of MCA-PSV has 100% sensitivity with a false-positive rate of 12% in the prediction of moderate to severe anemia in the non-hydropic fetus. Timely identification of significant fetal anemia is the basis for therapeutic intervention with intrauterine blood transfusion or delivery, depending on GA and thereby preventing fetal demise.
The Newborn in HDFN
When the fetus becomes a newborn it might still be suffering from anemia and the other pathophysiologic consequences of the persisting maternal antibody [54] present in the newborn. In most cases the fetal RBCs will carry maternal antibodies detectable by the DAT. We routinely determine the titer of free alloantibody in the plasma of the newborn and determine the fetal blood group antigen targeted by the maternal antibody. The latter is routinely done to assess the quality of laboratory work. FC-based measurement of fetal versus donor cells is decided in each case.
The laboratory should be aware of the importance of information being shared with the team of neonatologists providing postnatal care for the newborn. It should be remembered that laboratory investigation of the mother might still be relevant and can yield valuable information, for example examination for antibodies, phenotype, determination of titers, fetomaternal hemorrhage, especially in the RhD-positive women who have not been tested since the 1st trimester.
Further Preventive Measures to Avoid Alloimmunization
Prevention of alloimmunization due to transfusion in girls and women of premenopausal age, or under the age of 50 years, has been implemented in some countries by matching a limited number of RBC antigens. Basic matching of ABO and RhD blood groups is supplemented by supplying K-negative RBC components for premenopausal women in Denmark. Matching has been extended to routinely encompass Rhc and E in some countries [3].
A study on the effect of matching donor and recipient in IUT indicates that an efficient prevention of alloimmunization (64%) can be achieved by an extended phenotypic match: C, c, E, K, Fya, Jka, S [55]. Another study in ordinary transfusion recipients demonstrated that matching for C, c, E, K, Jka could prevent 78% of immunizations, and enhanced matching for C, c, E, K, Fya, Jka, Cw improved prevention to 83.4% of immunizations [56]. We have implemented matching for IUT for C, c, E, K, Fya, Jka with a pragmatic view for the available supply. However, our extensive genotyping of donors helps making matches possible by access to ample donor genotype information [57].
Platelet transfusion seems to be a source of alloimmunization that could be taken into consideration. Small amounts of RBCs in the platelet component are enough to immunize. We administer RhIg if, for logistical reasons, D-positive platelet or plasma components must be given to a female RhD-negative recipient of premenopausal age.
Perspectives for Optimization and Future Developments
Several studies have addressed the feasibility of screening all pregnant women for irregular antibodies in the 3rd trimester, not only RhD-negative women. First trimester screening of all pregnant women is already implemented in many health care systems. A 3rd trimester repeated screening of Rhc-negative women has been proposed. Focusing on individuals with a high risk of immunization would enhance cost benefit in comparison with screening all women [3, 8].
Some individuals develop alloantibodies after alloantigen exposure whereas others can be transfused repeatedly without being alloimmunized [58, 59]. Elucidation of the genetic background for individual propensity to develop alloantibodies as well as the genetic background for regulation of quantities of antibodies produced has been attempted by several groups [60, 61, 62]. Access to this information for pregnant women would potentially add useful guidance to the clinical risk assessment of a specific woman.
It has also been attempted to interfere with an established immune response by administration of peptides derived from the antigen to end the active production of antibody [63, 64]. Another approach is administration of non-destructive antibodies competing for the antigen to the alloimmunized woman [65, 66].
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
The authors have not received any funding relevant for this manuscript.
Author Contributions
All authors contributed to writing of the text. All authors have read and accepted the final version of the manuscript.
References
- 1.Bowman JM, Pollock JM, Penston LE. Fetomaternal transplacental hemorrhage during pregnancy and after delivery. Vox Sang. 1986;51((2)):117–21. doi: 10.1111/j.1423-0410.1986.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 2.de Haas M, Thurik FF, Koelewijn JM, van der Schoot CE. Haemolytic disease of the fetus and newborn. Vox Sang. 2015 Aug;109((2)):99–113. doi: 10.1111/vox.12265. [DOI] [PubMed] [Google Scholar]
- 3.Koelewijn JM, Vrijkotte TG, de Haas M, van der Schoot CE, Bonsel GJ. Risk factors for the presence of non-rhesus D red blood cell antibodies in pregnancy. BJOG. 2009 Apr;116((5)):655–64. doi: 10.1111/j.1471-0528.2008.01984.x. [DOI] [PubMed] [Google Scholar]
- 4.Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol. 2001 Aug;13((8)):993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 5.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337((6203)):184–7. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 6.Le Pichon JB, Riordan SM, Watchko J, Shapiro SM. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs) Curr Pediatr Rev. 2017;13((3)):199–209. doi: 10.2174/1573396313666170815100214. [DOI] [PubMed] [Google Scholar]
- 7.Luban NL. Hemolytic disease of the newborn: progenitor cells and late effects. N Engl J Med. 1998 Mar 19;338((12)):830–1. doi: 10.1056/NEJM199803193381210. [DOI] [PubMed] [Google Scholar]
- 8.Koelewijn JM, Vrijkotte TG, van der Schoot CE, Bonsel GJ, de Haas M. Effect of screening for red cell antibodies, other than anti-D, to detect hemolytic disease of the fetus and newborn: a population study in the Netherlands. Transfusion. 2008 May;48((5)):941–52. doi: 10.1111/j.1537-2995.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 9.Finning K, Martin P, Summers J, Daniels G. Fetal genotyping for the K (Kell) and Rh C, c, and E blood groups on cell-free fetal DNA in maternal plasma. Transfusion. 2007 Nov;47((11)):2126–33. doi: 10.1111/j.1537-2995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 10.Moise KJ. Red blood cell alloimmunization in pregnancy. Semin Hematol. 2005 Jul;42((3)):169–78. doi: 10.1053/j.seminhematol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Mari G, Deter RL, Carpenter RL, Rahman F, Zimmerman R, Moise KJ, Jr., et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med. 2000 Jan 6;342((1)):9–14. doi: 10.1056/NEJM200001063420102. [DOI] [PubMed] [Google Scholar]
- 12.Clausen FB. Lessons learned from the implementation of non-invasive fetal RHD screening. Expert Rev Mol Diagn. 2018 May;18((5)):423–31. doi: 10.1080/14737159.2018.1461562. [DOI] [PubMed] [Google Scholar]
- 13.van der Schoot CE, de Haas M, Clausen FB. Genotyping to prevent Rh disease: has the time come? Curr Opin Hematol. 2017 Nov;24((6)):544–50. doi: 10.1097/MOH.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 14.Bills VL, Soothill PW. Fetal blood grouping using cell free DNA − an improved service for RhD negative pregnant women. Transfus Apher Sci. 2014 Apr;50((2)):148–53. doi: 10.1016/j.transci.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Kent J, Farrell AM, Soothill P. Routine administration of Anti-D: the ethical case for offering pregnant women fetal RHD genotyping and a review of policy and practice. BMC Pregnancy Childbirth. 2014 Feb 25;14:87. doi: 10.1186/1471-2393-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faas BH, Beuling EA, Christiaens GC, von dem Borne AE, van der Schoot CE. Detection of fetal RHD-specific sequences in maternal plasma. Lancet. 1998 Oct 10;352((9135)):1196. doi: 10.1016/s0140-6736(05)60534-x. [DOI] [PubMed] [Google Scholar]
- 17.Lo YM, Hjelm NM, Fidler C, Sargent IL, Murphy MF, Chamberlain PF, et al. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med. 1998 Dec 10;339((24)):1734–8. doi: 10.1056/NEJM199812103392402. [DOI] [PubMed] [Google Scholar]
- 18.Alshehri AA, Jackson DE. Non-invasive prenatal fetal blood group genotype and its application in the management of hemolytic disease of fetus and newborn: systematic review and meta-analysis. Transfus Med Rev. 2021;35((2)):85–94. doi: 10.1016/j.tmrv.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Clausen FB. Integration of noninvasive prenatal prediction of fetal blood group into clinical prenatal care. Prenat Diagn. 2014 May;34((5)):409–15. doi: 10.1002/pd.4326. [DOI] [PubMed] [Google Scholar]
- 20.Clausen FB, Damkjær MB, Dziegiel MH. Noninvasive fetal RhD genotyping. Transfus Apher Sci. 2014 Apr;50((2)):154–62. doi: 10.1016/j.transci.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Daniels G, Finning K, Martin P, Massey E. Noninvasive prenatal diagnosis of fetal blood group phenotypes: current practice and future prospects. Prenat Diagn. 2009 Feb;29((2)):101–7. doi: 10.1002/pd.2172. [DOI] [PubMed] [Google Scholar]
- 22.Runkel B, Bein G, Sieben W, Sow D, Polus S, Fleer D. Targeted antenatal anti-D prophylaxis for RhD-negative pregnant women: a systematic review. BMC Pregnancy Childbirth. 2020 Feb 7;20((1)):83. doi: 10.1186/s12884-020-2742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Llewellyn A, Walker R, Harden M, Saramago P, Griffin S, et al. High-throughput, non-invasive prenatal testing for fetal rhesus D status in RhD-negative women: a systematic review and meta-analysis. BMC Med. 2019 Feb 14;17((1)):37. doi: 10.1186/s12916-019-1254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu YJ, Zheng YR, Li L, Zhou H, Liao X, Guo JX, et al. Diagnostic accuracy of non-invasive fetal RhD genotyping using cell-free fetal DNA: a meta analysis. J Matern Fetal Neonatal Med. 2014 Dec;27((18)):1839–44. doi: 10.3109/14767058.2014.882306. [DOI] [PubMed] [Google Scholar]
- 25.Clausen FB, Hellberg A, Bein G, Bugert P, Schwartz D, Drnovsek TD, et al. Recommendation for validation and quality assurance of non-invasive prenatal testing for foetal blood groups and implications for IVD risk classification according to EU regulations. Vox Sang. 2021 doi: 10.1111/vox.13172. doi: 10.1111/vox.13172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clausen FB, Christiansen M, Steffensen R, Jørgensen S, Nielsen C, Jakobsen MA, et al. Report of the first nationally implemented clinical routine screening for fetal RHD in D− pregnant women to ascertain the requirement for antenatal RhD prophylaxis. Transfusion. 2012 Apr;52((4)):752–8. doi: 10.1111/j.1537-2995.2011.03362.x. [DOI] [PubMed] [Google Scholar]
- 27.Clausen FB, Rieneck K, Krog GR, Bundgaard BS, Dziegiel MH. Noninvasive antenatal screening for fetal RHD in RhD negative women to guide targeted anti-D prophylaxis. Methods Mol Biol. 2019;1885:347–59. doi: 10.1007/978-1-4939-8889-1_23. [DOI] [PubMed] [Google Scholar]
- 28.Clausen FB, Steffensen R, Christiansen M, Rudby M, Jakobsen MA, Jakobsen TR, et al. Routine noninvasive prenatal screening for fetal RHD in plasma of RhD-negative pregnant women − 2 years of screening experience from Denmark. Prenat Diagn. 2014 Oct;34((10)):1000–5. doi: 10.1002/pd.4419. [DOI] [PubMed] [Google Scholar]
- 29.Clausen FB, Krog GR, Rieneck K, Råsmark EE, Dziegiel MH. Evaluation of two real-time multiplex PCR screening assays detecting fetal RHD in plasma from RhD negative women to ascertain the requirement for antenatal RhD prophylaxis. Fetal Diagn Ther. 2011;29((2)):155–63. doi: 10.1159/000321347. [DOI] [PubMed] [Google Scholar]
- 30.Clausen FB, Urhammer E, Rieneck K, Krog GR, Nielsen LK, Dziegiel MH. How to evaluate PCR assays for the detection of low-level DNA. APMIS. 2015 Sep;123((9)):731–9. doi: 10.1111/apm.12405. [DOI] [PubMed] [Google Scholar]
- 31.de Haas M, Thurik FF, van der Ploeg CP, Veldhuisen B, Hirschberg H, Soussan AA, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016 Nov 7;355:i5789. doi: 10.1136/bmj.i5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haimila K, Sulin K, Kuosmanen M, Sareneva I, Korhonen A, Natunen S, et al. Targeted antenatal anti-D prophylaxis program for RhD-negative pregnant women − outcome of the first two years of a national program in Finland. Acta Obstet Gynecol Scand. 2017 Oct;96((10)):1228–33. doi: 10.1111/aogs.13191. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MPS, Damkjaer MB, Clausen FB, Ali HA, Hare KJ, Dziegiel MH, et al. Targeted Rhesus immunoglobulin for RhD-negative women undergoing an induced abortion: a clinical pilot study. Acta Obstet Gynecol Scand. 2019 Sep;98((9)):1164–71. doi: 10.1111/aogs.13606. [DOI] [PubMed] [Google Scholar]
- 34.Rieneck K, Bak M, Jønson L, Clausen FB, Krog GR, Tommerup N, et al. Next-generation sequencing: proof of concept for antenatal prediction of the fetal Kell blood group phenotype from cell-free fetal DNA in maternal plasma. Transfusion. 2013 Nov;53((11 Suppl 2)):2892–8. doi: 10.1111/trf.12172. [DOI] [PubMed] [Google Scholar]
- 35.Rieneck K, Clausen FB, Dziegiel MH. Next-generation sequencing for antenatal prediction of KEL1 blood group status. Methods Mol Biol. 2015;1310:115–21. doi: 10.1007/978-1-4939-2690-9_10. [DOI] [PubMed] [Google Scholar]
- 36.Rieneck K, Clausen FB, Dziegiel MH. Noninvasive antenatal determination of fetal blood group using next-generation sequencing. Cold Spring Harb Perspect Med. 2015 Oct 28;6((1)):a023093. doi: 10.1101/cshperspect.a023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieneck K, Egeberg Hother C, Clausen FB, Jakobsen MA, Bergholt T, Hellmuth E, et al. Next generation sequencing-based fetal abo blood group prediction by analysis of cell-free DNA from maternal plasma. Transfus Med Hemother. 2020 Feb;47((1)):45–53. doi: 10.1159/000505464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Brien H, Hyland C, Schoeman E, Flower R, Daly J, Gardener G. Non-invasive prenatal testing (NIPT) for fetal Kell, Duffy and Rh blood group antigen prediction in alloimmunised pregnant women: power of droplet digital PCR. Br J Haematol. 2020 May;189((3)):e90–4. doi: 10.1111/bjh.16500. [DOI] [PubMed] [Google Scholar]
- 39.Orzińska A, Guz K, Mikula M, Kluska A, Balabas A, Ostrowski J, et al. Prediction of fetal blood group and platelet antigens from maternal plasma using next-generation sequencing. Transfusion. 2019 Mar;59((3)):1102–7. doi: 10.1111/trf.15116. [DOI] [PubMed] [Google Scholar]
- 40.Delaney M, Matthews DC. Hemolytic disease of the fetus and newborn: managing the mother, fetus, and newborn. Hematology Am Soc Hematol Educ Program. 2015;2015:146–51. doi: 10.1182/asheducation-2015.1.146. [DOI] [PubMed] [Google Scholar]
- 41.Donneborg ML, Hansen BM, Vandborg PK, Rodrigo-Domingo M, Ebbesen F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in Denmark during the years 2000-2015. J Perinatol. 2020 Feb;40((2)):194–202. doi: 10.1038/s41372-019-0566-8. [DOI] [PubMed] [Google Scholar]
- 42.Ziprin JH, Payne E, Hamidi L, Roberts I, Regan F. ABO incompatibility due to immunoglobulin G anti-B antibodies presenting with severe fetal anaemia. Transfus Med. 2005 Feb;15((1)):57–60. doi: 10.1111/j.1365-3148.2005.00549.x. [DOI] [PubMed] [Google Scholar]
- 43.Zonneveld R, van der Meer-Kapelle L, Sylva M, Brand A, Zijlstra M, Schonewille H. Severe fetal hemolysis and cholestasis due to high-titer maternal IgG anti-A antibodies. Pediatrics. 2019 Apr;143((4)):e20182859. doi: 10.1542/peds.2018-2859. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan M, Hammerman C, Vreman HJ, Wong RJ, Stevenson DK. Hemolysis and hyperbilirubinemia in antiglobulin positive, direct ABO blood group heterospecific neonates. J Pediatr. 2010 Nov;157((5)):772–7. doi: 10.1016/j.jpeds.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonnell M, Hannam S, Devane SP. Hydrops fetalis due to ABO incompatibility. Arch Dis Child Fetal Neonatal Ed. 1998 May;78((3)):F220–1. doi: 10.1136/fn.78.3.f220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarici SU, Yurdakök M, Serdar MA, Oran O, Erdem G, Tekinalp G, et al. An early (sixth-hour) serum bilirubin measurement is useful in predicting the development of significant hyperbilirubinemia and severe ABO hemolytic disease in a selective high-risk population of newborns with ABO incompatibility. Pediatrics. 2002 Apr;109((4)):e53. doi: 10.1542/peds.109.4.e53. [DOI] [PubMed] [Google Scholar]
- 47.Bakkeheim E, Bergerud U, Schmidt-Melbye AC, Akkok CA, Liestol K, Fugelseth D, et al. Maternal IgG anti-A and anti-B titres predict outcome in ABO-incompatibility in the neonate. Acta Paediatr. 2009 Dec;98((12)):1896–901. doi: 10.1111/j.1651-2227.2009.01478.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen JY, Ling UP. Prediction of the development of neonatal hyperbilirubinemia in ABO incompatibility. Zhonghua Yi Xue Za Zhi. 1994 Jan;53((1)):13–8. [PubMed] [Google Scholar]
- 49.Krog GR, Donneborg ML, Hansen BM, Lorenzen H, Clausen FB, Jensen KV, et al. Prediction of ABO hemolytic disease of the newborn using pre- and perinatal quantification of maternal anti-A/anti-B IgG titer. Pediatr Res. 2021;90((1)):74–81. doi: 10.1038/s41390-020-01232-5. [DOI] [PubMed] [Google Scholar]
- 50.Dziegiel MH, Nielsen LK, Berkowicz A. Detecting fetomaternal hemorrhage by flow cytometry. Curr Opin Hematol. 2006 Nov;13((6)):490–5. doi: 10.1097/01.moh.0000245687.09215.c4. [DOI] [PubMed] [Google Scholar]
- 51.Reyes F, Gourdin MF, Lejonc JL, Cartron JP, Gorius JB, Dreyfus B. The heterogeneity of erythrocyte antigen distribution in human normal phenotypes: an immunoelectron microscopy study. Br J Haematol. 1976 Dec;34((4)):613–21. doi: 10.1111/j.1365-2141.1976.tb03608.x. [DOI] [PubMed] [Google Scholar]
- 52.Dziegiel MH, Hansen MH, Haedersdal S, Barrett AN, Rieneck K, Main KM, et al. Blood chimerism in dizygotic monochorionic twins during 5 years observation. Am J Transplant. 2017 Oct;17((10)):2728–32. doi: 10.1111/ajt.14318. [DOI] [PubMed] [Google Scholar]
- 53.Dooren MC, Kuijpers RW, Joekes EC, Huiskes E, Goldschmeding R, Overbeeke MA, et al. Protection against immune haemolytic disease of newborn infants by maternal monocyte-reactive IgG alloantibodies (anti-HLA-DR) Lancet. 1992 May 2;339((8801)):1067–70. doi: 10.1016/0140-6736(92)90661-l. [DOI] [PubMed] [Google Scholar]
- 54.Gijtenbeek M, Lopriore E, Steggerda SJ, Te Pas AB, Oepkes D, Haak MC. Persistent pulmonary hypertension of the newborn after fetomaternal hemorrhage. Transfusion. 2018 Dec;58((12)):2819–24. doi: 10.1111/trf.14932. [DOI] [PubMed] [Google Scholar]
- 55.Schonewille H, Klumper FJ, van de Watering LM, Kanhai HH, Brand A. High additional maternal red cell alloimmunization after Rhesus- and K-matched intrauterine intravascular transfusions for hemolytic disease of the fetus. Am J Obstet Gynecol. 2007 Feb;196((2)):143–6. doi: 10.1016/j.ajog.2006.10.895. [DOI] [PubMed] [Google Scholar]
- 56.Evers D, Middelburg RA, de Haas M, Zalpuri S, de Vooght KM, van de Kerkhof D, et al. Red-blood-cell alloimmunisation in relation to antigens' exposure and their immunogenicity: a cohort study. Lancet Haematol. 2016 Jun;3((6)):e284–92. doi: 10.1016/S2352-3026(16)30019-9. [DOI] [PubMed] [Google Scholar]
- 57.Krog GR, Rieneck K, Clausen FB, Steffensen R, Dziegiel MH. Blood group genotyping of blood donors: validation of a highly accurate routine method. Transfusion. 2019 Oct;59((10)):3264–74. doi: 10.1111/trf.15474. [DOI] [PubMed] [Google Scholar]
- 58.Hendrickson JE, Delaney M. Hemolytic disease of the fetus and newborn: modern practice and future investigations. Transfus Med Rev. 2016 Oct;30((4)):159–64. doi: 10.1016/j.tmrv.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Stack G, Tormey CA. Estimating the immunogenicity of blood group antigens: a modified calculation that corrects for transfusion exposures. Br J Haematol. 2016 Oct;175((1)):154–60. doi: 10.1111/bjh.14175. [DOI] [PubMed] [Google Scholar]
- 60.Jonsson S, Sveinbjornsson G, de Lapuente Portilla AL, Swaminathan B, Plomp R, Dekkers G, et al. Identification of sequence variants influencing immunoglobulin levels. Nat Genet. 2017 Aug;49((8)):1182–91. doi: 10.1038/ng.3897. [DOI] [PubMed] [Google Scholar]
- 61.Tan JCG, Yuan FF, Daley J, Marks K, Flower RL, Dyer WB. D-immunized blood donors who are female and who possess at least one HLA-DRB1*15 allele show a propensity for high serum RhIG production. Transfusion. 2018 May;58((5)):1182–8. doi: 10.1111/trf.14584. [DOI] [PubMed] [Google Scholar]
- 62.Verduin EP, Brand A, van de Watering LM, Roelen DL, Kanhai HH, Doxiadis, et al. The HLA-DRB1*15 phenotype is associated with multiple red blood cell and HLA antibody responsiveness. Transfusion. 2016 Jul;56((7)):1849–56. doi: 10.1111/trf.13648. [DOI] [PubMed] [Google Scholar]
- 63.Hall AM, Cairns LS, Altmann DM, Barker RN, Urbaniak SJ. Immune responses and tolerance to the RhD blood group protein in HLA-transgenic mice. Blood. 2005 Mar 1;105((5)):2175–9. doi: 10.1182/blood-2004-04-1554. [DOI] [PubMed] [Google Scholar]
- 64.Hall LS, Hall AM, Pickford W, Vickers MA, Urbaniak SJ, Barker RN. Combination peptide immunotherapy suppresses antibody and helper T-cell responses to the RhD protein in HLA-transgenic mice. Haematologica. 2014 Mar;99((3)):588–96. doi: 10.3324/haematol.2012.082081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathiesen L, Nielsen LK, Andersen JT, Grevys A, Sandlie I, Michaelsen TE, et al. Maternofetal transplacental transport of recombinant IgG antibodies lacking effector functions. Blood. 2013 Aug 15;122((7)):1174–81. doi: 10.1182/blood-2012-12-473843. [DOI] [PubMed] [Google Scholar]
- 66.Nielsen LK, Green TH, Sandlie I, Michaelsen TE, Dziegiel MH. In vitro assessment of recombinant, mutant immunoglobulin G anti-D devoid of hemolytic activity for treatment of ongoing hemolytic disease of the fetus and newborn. Transfusion. 2008 Jan;48((1)):12–9. doi: 10.1111/j.1537-2995.2007.01474.x. [DOI] [PubMed] [Google Scholar]