Abstract
BACKGROUND
Additional interventions are needed to reduce the morbidity and mortality caused by malaria.
METHODS
We conducted a two-part, phase 1 clinical trial to assess the safety and pharmacokinetics of CIS43LS, an antimalarial monoclonal antibody with an extended half-life, and its efficacy against infection with Plasmodium falciparum. Part A of the trial assessed the safety, initial side-effect profile, and pharmacokinetics of CIS43LS in healthy adults who had never had malaria. Participants received CIS43LS subcutaneously or intravenously at one of three escalating dose levels. A subgroup of participants from Part A continued to Part B, and some received a second CIS43LS infusion. Additional participants were enrolled in Part B and received CIS43LS intravenously. To assess the protective efficacy of CIS43LS, some participants underwent controlled human malaria infection in which they were exposed to mosquitoes carrying P. falciparum sporozoites 4 to 36 weeks after administration of CIS43LS.
RESULTS
A total of 25 participants received CIS43LS at a dose of 5 mg per kilogram of body weight, 20 mg per kilogram, or 40 mg per kilogram, and 4 of the 25 participants received a second dose (20 mg per kilogram regardless of initial dose). No safety concerns were identified. We observed dose-dependent increases in CIS43LS serum concentrations, with a half-life of 56 days. None of the 9 participants who received CIS43LS, as compared with 5 of 6 control participants who did not receive CIS43LS, had parasitemia according to polymerase-chain-reaction testing through 21 days after controlled human malaria infection. Two participants who received 40 mg per kilogram of CIS43LS and underwent controlled human malaria infection approximately 36 weeks later had no parasitemia, with serum concentrations of CIS43LS of 46 and 57 μg per milliliter at the time of controlled human malaria infection.
CONCLUSIONS
Among adults who had never had malaria infection or vaccination, administration of the long-acting monoclonal antibody CIS43LS prevented malaria after controlled infection. (Funded by the National Institute of Allergy and Infectious Diseases; VRC 612 ClinicalTrials.gov number, NCT04206332.)
Malaria is a mosquito-borne parasitic disease caused by Plasmodium falciparum that affects approximately 200 to 400 million people each year, resulting in nearly 400,000 annual deaths and disproportionately affecting children in sub-Saharan Africa.1 Although public health measures such as insecticide-treated bed nets and antimalarial drugs led to a 50 to 75% reduction in global malaria cases in the period from 2000 to 2015,2 the incidence of malaria is now increasing in many areas despite these interventions.1 With respect to vaccines, three doses of RTS,S, a protein subunit vaccine adjuvanted with AS01, conferred approximately 50% protection against clinical infection at 1 year and 28% protection over 4 years in phase 3 studies involving children 5 to 17 months of age.3–6 Given the health and economic burden attributable to malaria, additional countermeasures are needed to better control and possibly eliminate this disease.7,8
Antibodies can prevent malaria by neutralizing the infectious P. falciparum sporozoites in the skin and blood before they can infect hepatocytes in the liver.9–11 The P. falciparum circumsporozoite protein is the most abundant P. falciparum sporozoite surface protein and is required for parasite motility and invasion of hepatocytes, making it a critical antigenic target for antibody neutralization and subunit vaccine development.12–17 The P. falciparum circumsporozoite protein has three major domains: an N-terminal domain, a central region composed of repeating tetrapeptides characterized by NANP repeats, and a C-terminal region. Although the majority of neutralizing monoclonal antibodies against P. falciparum circumsporozoite protein bind the immunodominant central NANP repeats,18–21 we previously identified a new site of vulnerability that spans the NPDP tetrapeptide and is located at the junction of the N-terminal and central repeat regions. This site was defined with the use of binding analyses of a new human monoclonal antibody, CIS43,22 which was isolated from a participant in a clinical trial who was immunized with an attenuated P. falciparum whole-sporozoite vaccine (Sanaria).23 CIS43 exhibited preferential specificity for the junctional NPDP epitope and was highly protective in several preclinical mouse models of malaria infection.22 The junctional NPDP epitope was highly conserved in 99.9% of more than 6500 P. falciparum field isolates analyzed.22 Before it was evaluated in humans, CIS43 was modified to CIS43LS24 by means of site-directed mutagenesis of its Fc region, which converted methionine to leucine and asparagine to serine to prolong plasma half-life through increased neonatal Fc receptor–mediated antibody recirculation.25 Here, we report the results of a phase 1 clinical trial conducted to assess the safety, initial side-effect profile, pharmacokinetics, and protective efficacy of CIS43LS in healthy adults who had not previously had malaria or received a vaccine for malaria.
METHODS
TRIAL DESIGN AND PARTICIPANTS
VRC 612 was a two-part, first-in-human, open-label, phase 1, dose-escalation clinical trial. The primary objectives of the trial were to evaluate the safety and initial side-effect profile of CIS43LS. Secondary objectives were to assess the pharmacokinetic properties and efficacy of CIS43LS in preventing malaria after controlled human malaria infection. Eligible participants were healthy adults 18 to 50 years of age who had not had previous malaria infection or vaccination. Full details of the inclusion and exclusion criteria are provided in the protocol, available with the full text of this article at NEJM.org.
TRIAL OVERSIGHT
The trial was designed, funded, and conducted by the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), at the NIH Clinical Center. Controlled human malaria infection was conducted at the U.S. Army facility at Walter Reed Army Institute of Research (WRAIR) in Silver Spring, Maryland. The NIH institutional review board reviewed and approved the trial protocol. All participants provided written informed consent, and the trial followed the Department of Health and Human Services guidelines for the protection of human research participants. Data were gathered and analyzed by the VRC and WRAIR. All the authors vouch for the accuracy of the data and analyses and for the adherence of the trial to the protocol.
TRIAL PRODUCT
CIS43LS is a human IgG1 monoclonal antibody that is derived from a Chinese hamster ovary DG44 stably transfected clonal cell line.24 It was manufactured according to Current Good Manufacturing Practices by the Vaccine Clinical Materials Program, operated under contract with Leidos Biomedical Research, and vialed in a buffered formulation at a concentration of 100 mg per milliliter.
TRIAL PROCEDURES
CIS43LS was administered intravenously over 30 minutes at a dose of 5 mg per kilogram of body weight, 20 mg per kilogram, or 40 mg per kilogram. Participants who received subcutaneous injections received 5 mg per kilogram, with the total dose divided into up to four abdominal injections on the basis of the weight of the participant. Individual injections did not exceed 2.5 ml. All participants were observed for 2 to 4 hours after administration of CIS43LS.
Two escalations in the intravenous doses were performed in Part A of the trial to assess safety. The first group of participants who were enrolled received a single dose of 5 mg per kilogram. A single dose of 20 mg per kilogram was similarly assessed in separate participants before a single dose of 40 mg per kilogram was administered to a final group of participants. Safety data included participant-reported solicited adverse events that occurred through 7 days after each administration as well as clinical and laboratory-based assessments at protocol-specified trial visits. Unsolicited adverse events were recorded for 28 days after CIS43LS administration and for an additional 28 days after controlled human malaria infection and were graded according to a modified Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 2.1. Serious adverse events and new chronic medical conditions were recorded for the entire duration of the trial.
Participants were followed for 6 months after the final CIS43LS administration. Participants were able to choose if they wanted to volunteer as control participants — they did not receive CIS43LS, but they underwent controlled human malaria infection and were followed for 8 weeks after the controlled infection.
CONTROLLED HUMAN MALARIA INFECTION
Participants were exposed to bites on the forearm from Anopheles stephensi mosquitoes infected with P. falciparum (3D7 strain). The mosquitoes met standard infectivity criteria as previously described.23 Outpatient monitoring was performed by means of two telephone calls in the first 7 days after infection challenge, followed by clinic visits on days 7 through 18 and on day 21 to assess for parasitemia with standard polymerase-chain-reaction (PCR) methods.26 Parasitemia (i.e., malaria infection) was defined as a single positive PCR result. Participants were considered protected if they remained negative for parasitemia through day 21 after infection. Directly observed treatment with 1 g of atovaquone and 400 mg of proguanil hydrochloride was administered for 3 consecutive days, beginning at the time parasitemia was confirmed or on day 21 if the participant had not already been treated.
PHARMACOKINETICS
Serum concentrations of CIS43LS were quantified on the Meso Scale Discovery platform with the use of a CIS43 anti-idiotype antibody24 at prespecified time points. Details regarding the methods are provided in the Supplementary Appendix, available at NEJM.org. Samples for pharmacokinetic analysis were obtained before CIS43LS administration and up to 36 weeks after administration. Descriptive statistics for the maximum concentration (Cmax) and for the concentrations on day 7 and at weeks 4 and 24 were calculated on the basis of observed data. Additional pharmacokinetic analyses are described in the Supplemental Methods section of the Supplementary Appendix.
STATISTICAL ANALYSIS
The target sample size was determined on the basis of the probability of observing serious adverse events. The efficacy analysis included all enrolled participants who received CIS43LS and underwent controlled human malaria infection. The primary efficacy analysis was performed with the use of a Barnard test to assess the percentage of participants who had malaria infection. The secondary efficacy analysis was performed with the use of a log-rank test to compare the time to parasitemia among participants who received CIS43LS with that among control participants. The salivary gland scores for the mosquitoes used in controlled infections are reported, along with the median values and interquartile ranges.
RESULTS
PARTICIPANTS
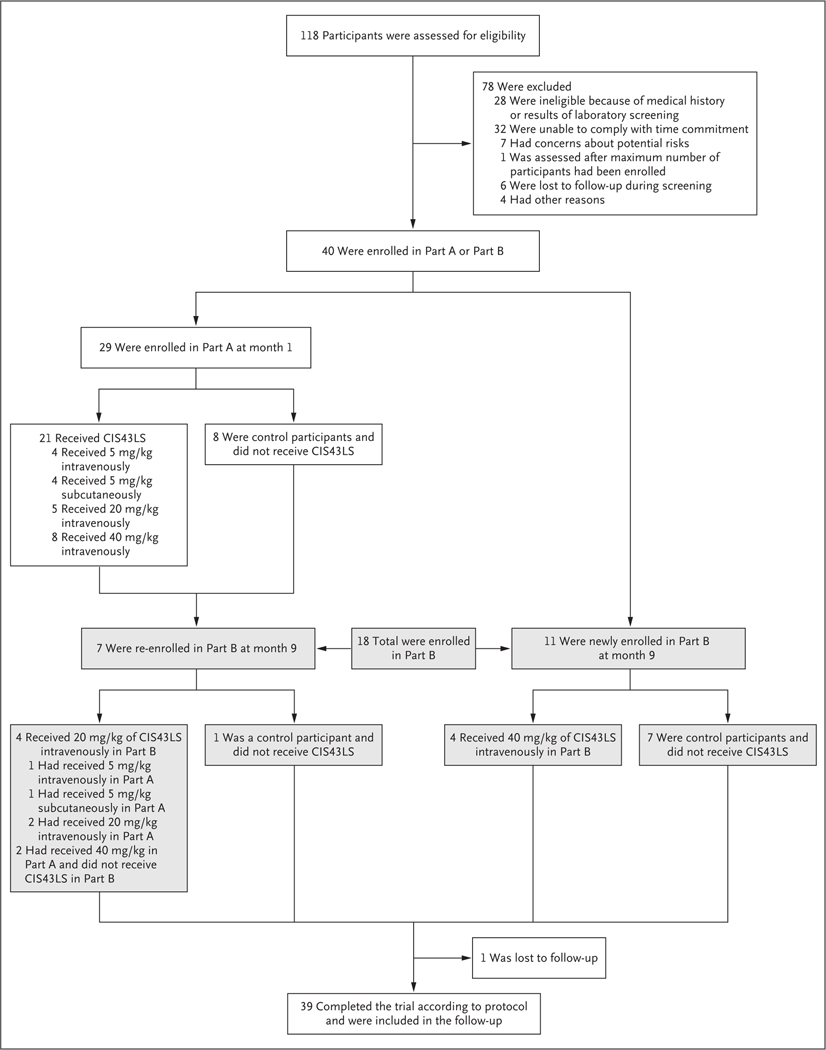
From January 7 to March 5, 2020, a total of 29 participants were enrolled in Part A of the trial (Fig. 1). CIS43LS was administered to 21 participants between January 7 and March 2, 2020; 4 participants received CIS43LS intravenously at a dose of 5 mg per kilogram, 4 received a subcutaneous dose of 5 mg per kilogram, 5 received an intravenous dose of 20 mg per kilogram, and 8 received an intravenous dose of 40 mg per kilogram. A total of 8 participants were enrolled to be control participants and did not receive CIS43LS. Controlled human malaria infection in Part A was originally scheduled for March 17, 2020, but was canceled because of restrictions related to coronavirus disease 2019 (Covid-19). Participants in Part A were followed for safety and pharmacokinetic assessments through in-person and remote visits until August 17, 2020.
Figure 1. Participants and Administration of CIS43LS.
The VRC 612 trial was conducted in two parts. Because of restrictions related to coronavirus disease 2019, the controlled human malaria infection that was originally planned for Part A was canceled, and the trial was modified. Of the 18 participants enrolled in Part B, 15 underwent controlled infection in month 10 (October 2020): 9 of these participants had received CIS43LS and 6 were control participants.
From September 8 to October 16, 2020, a total of 18 participants were enrolled in Part B; 7 participants from Part A were re-enrolled and 11 were newly enrolled. Among the 18 participants in Part B, 10 received CIS43LS in Part A, Part B, or both, and 8 served as control participants. Among the 11 newly enrolled participants, 4 received 40 mg of CIS43LS intravenously, and 7 served as control participants. Among the 7 participants from Part A who were re-enrolled, 4 received CIS43LS at a dose of 20 mg per kilogram intravenously in Part B (1 had previously received 5 mg per kilogram intravenously, 1 had received 5 mg per kilogram subcutaneously, and 2 had received 20 mg per kilogram intravenously), and 2 participants who had received 40 mg per kilogram intravenously in Part A underwent controlled human malaria infection but did not receive an additional dose in Part B.
Controlled human malaria infection was administered to 15 participants (9 who had received CIS43LS and 6 control participants) on October 20, 2020. The 21-day monitoring for parasitemia concluded on November 10, 2020. One participant who received 40 mg per kilogram intravenously in Part B did not undergo controlled infection because of a concomitant illness. Participants in Part B were followed through March 2021. Maximum enrollment was not met in Part B because of Covid-19–related restrictions. The demographic characteristics of the participants are provided in Table S1 in the Supplementary Appendix.
SAFETY
No safety concerns were identified (Table 1). When present, solicited adverse events were mild to moderate in severity. There were no infusion-related reactions, and no serious adverse events were attributed to CIS43LS. Unsolicited adverse events that were attributed to CIS43LS only occurred among participants who received 5 mg per kilogram intravenously, and the events were all of grade 1. Single events of dizziness, transient asymptomatic neutropenia, and elevation in creatinine level (an increase of 1.2 to 1.4 mg per deciliter [106 to 124 μmol per liter]) were assessed as being related to CIS43LS. All adverse events resolved without intervention or residual effects. One participant had a serious adverse event unrelated to CIS43LS that consisted of unscheduled hospital admission for treatment of a perirectal abscess.
Table 1.
Maximum Local and Systemic Reactogenicity after Administration of CIS43LS According to Dose and Route of Administration.*
| Symptom and Severity | 5 mg/kg IV (N = 4) | 5 mg/kg SC (N = 4) | 20 mg/kg IV (N = 9)† | 40 mg/kg IV (N = 12) | All Groups (N = 29) |
|---|---|---|---|---|---|
| number of participants (percent) | |||||
| Local reactogenicity | |||||
| Pain and tenderness | |||||
| None | 4 (100) | 1 (25) | 7 (78) | 10 (83) | 22 (76) |
| Mild | 0 | 3 (75) | 2 (22) | 2 (17) | 7 (24) |
| Pruritus | |||||
| None | 4 (100) | 4 (100) | 9 (100) | 12 (100) | 29 (100) |
| Swelling | |||||
| None | 4 (100) | 4 (100) | 9 (100) | 12 (100) | 29 (100) |
| Redness | |||||
| None | 4 (100) | 4 (100) | 9 (100) | 12 (100) | 29 (100) |
| Bruising | |||||
| None | 4 (100) | 4 (100) | 9 (100) | 11 (92) | 28 (97) |
| Moderate | 0 | 0 | 0 | 1 (8) | 1 (3) |
| Any local symptom | |||||
| None | 4 (100) | 1 (25) | 7 (78) | 10 (83) | 22 (76) |
| Mild | 0 | 3 (75) | 2 (22) | 1 (8) | 6 (21) |
| Moderate | 0 | 0 | 0 | 1 (8) | 1 (3) |
| Systemic reactogenicity | |||||
| Malaise | |||||
| None | 4 (100) | 4 (100) | 7 (78) | 11 (92) | 26 (90) |
| Mild | 0 | 0 | 2 (22) | 0 | 2 (7) |
| Moderate | 0 | 0 | 0 | 1 (8) | 1 (3) |
| Muscle aches | |||||
| None | 4 (100) | 2 (50) | 8 (89) | 10 (83) | 24 (83) |
| Mild | 0 | 2 (50) | 1 (11) | 2 (17) | 5 (17) |
| Headache | |||||
| None | 3 (75) | 3 (75) | 7 (78) | 9 (75) | 22 (76) |
| Mild | 0 | 1 (25) | 2 (22) | 3 (25) | 6 (21) |
| Moderate | 1 (25) | 0 | 0 | 0 | 1 (3) |
| Chills | |||||
| None | 4 (100) | 4 (100) | 9 (100) | 12 (100) | 29 (100) |
| Nausea | |||||
| None | 3 (75) | 4 (100) | 8 (89) | 12 (100) | 27 (93) |
| Mild | 0 | 0 | 1 (11) | 0 | 1 (3) |
| Moderate | 1 (25) | 0 | 0 | 0 | 1 (3) |
| Joint pain | |||||
| None | 4 (100) | 4 (100) | 9 (100) | 11 (92) | 28 (97) |
| Mild | 0 | 0 | 0 | 1 (8) | 1 (3) |
| Elevated temperature | |||||
| None | 4 (100) | 4 (100) | 9 (100) | 11 (92) | 28 (97) |
| Mild | 0 | 0 | 0 | 1 (8) | 1 (3) |
| Any systemic symptom | |||||
| None | 3 (75) | 1 (25) | 5 (56) | 6 (50) | 15 (52) |
| Mild | 0 | 3 (75) | 4 (44) | 5 (42) | 12 (41) |
| Moderate | 1 (25) | 0 | 0 | 1 (8) | 2 (7) |
IV denotes intravenous, and SC subcutaneous.
Data are included for 4 participants who received a second dose of CIS43LS at 20 mg per kilogram of body weight in Part B.
PHARMACOKINETIC ASSESSMENTS
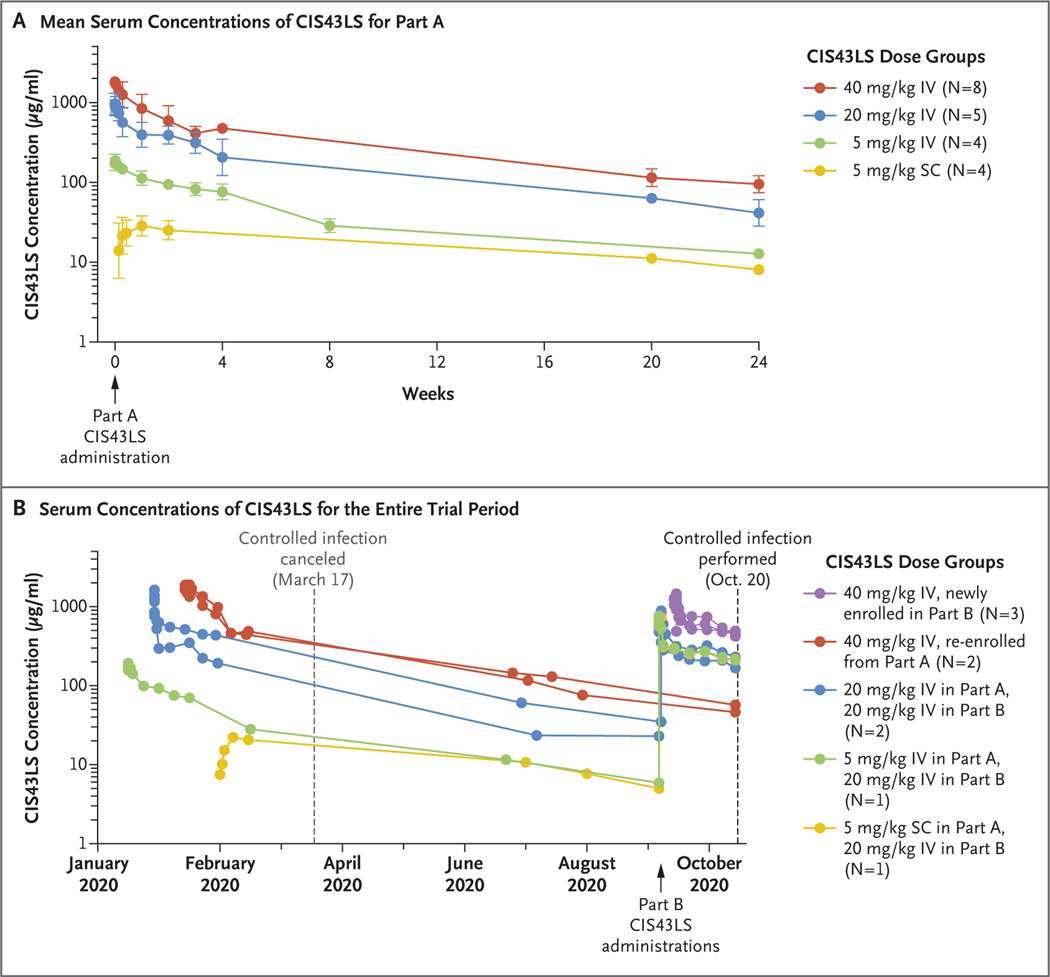
CIS43LS displayed dose linearity, expected distribution and elimination phases, and persistent concentrations in serum over several months (Figs. 2 and S1). Cmax occurred during the immediate postinfusion period among participants who received CIS43LS intravenously. After subcutaneous injection, absorption continued to at least day 7, and Cmax could not be fully calculated because of Covid-19–related interruptions in sample collection. The mean (±SD) Cmax was 198.4±28.2 μg per milliliter among participants who received 5 mg per kilogram intravenously, 934.6±292.6 μg per milliliter among those who received 20 mg per kilogram intravenously, and 1764.4±259.6 μg per milliliter among those who received 40 mg per kilogram intravenously. The mean serum concentrations at day 7 in these respective groups were 114.3±25.2, 356.1±118.6, and 825.3±293.1 μg per milliliter and remained detectable to week 24 at 12.8±1.0, 43.5±14.9, and 96.8±23.8 μg per milliliter.
Figure 2. Serum Concentrations of CIS43LS.
Panel A shows the mean serum concentrations of CIS43LS among participants in Part A. The geometric mean titers with standard deviations (indicated by I bars) are shown for each dose group after a single administration of CIS43LS (at week 0). The dose, route (intravenous [IV] or subcutaneous [SC]), and number of participants are specified in the key. Panel B shows the serum concentrations of CIS43LS across Parts A and B over time for individual participants who underwent controlled human malaria infection. Controlled infection in Part A had been planned for March 17, 2020, but was canceled. Participants underwent controlled infection in Part B on October 20, 2020.
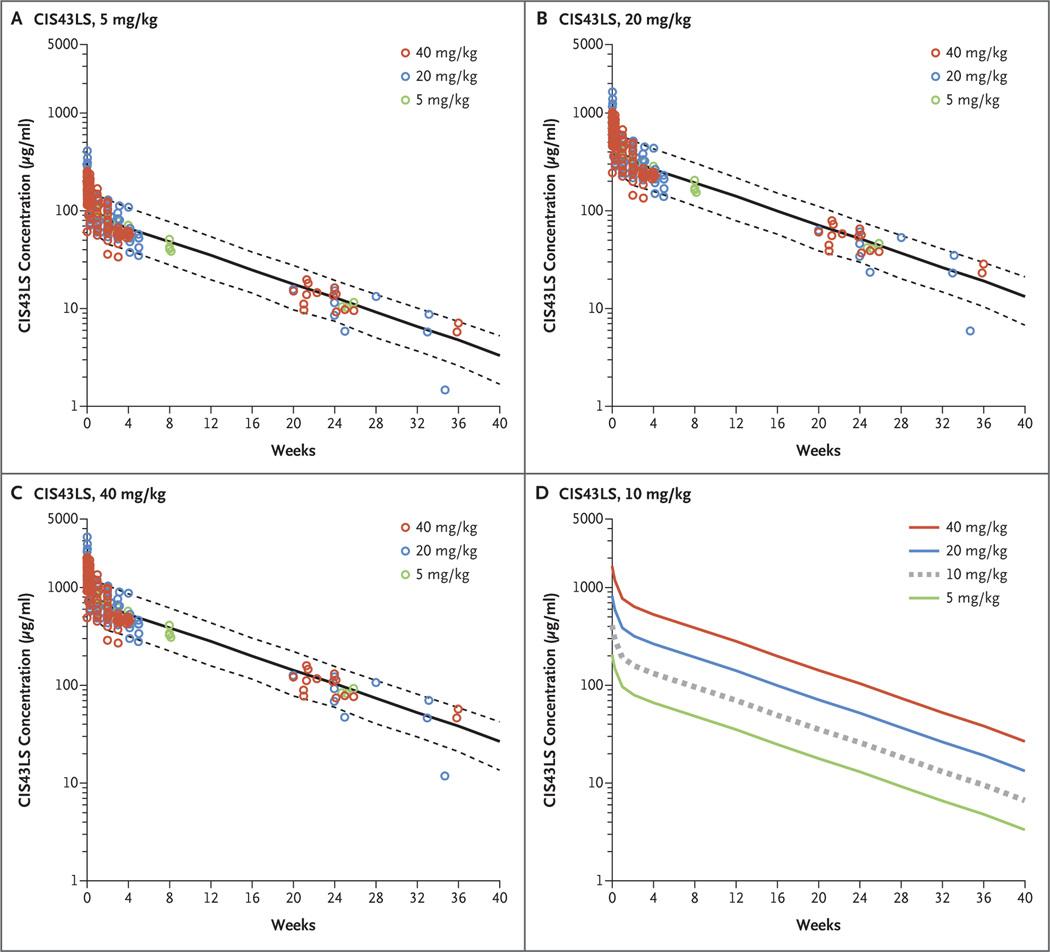
Quantification data for serum CIS43LS concentrations were used to develop a population pharmacokinetic model that was used for both predictive modeling and estimation of CIS43LS pharmacokinetic variables. According to the population pharmacokinetic model, the overall estimate of CIS43LS clearance was 44.2 ml per deciliter (90% confidence interval [CI], 39.6 to 47.9), the volume of distribution (Vdss) was 3.45 liters (90% CI, 3.25 to 4.13), and the half-life (T1/2β) was 56 days (90% CI, 51.6 to 77.0) (Table S2). The results of the population pharmacokinetic model of CIS43LS serum concentration after administration through approximately 40 weeks were similar to those of experimentally measured CIS43LS serum concentrations acquired over this period (Figs. 3 and S2). No dose effects on clearance or volumes of distribution were seen. Experimentally measured serum concentrations that were normalized to intravenous doses of 5 mg per kilogram, 20 mg per kilogram, and 40 mg per kilogram were equally well fit to the observed data across dose levels and lay within the 90% prediction interval. We performed simulations of serum concentrations after administration of the three intravenous dose levels and included a hypothetical dose of 10 mg per kilogram for comparison (Fig. 3); these data may be useful for the clinical design of future trials of CIS43LS.
Figure 3. Predictions for Serum Concentrations after Intravenous Administration of CIS43LS.
Panels A, B, and C show the predicted median serum concentrations of CIS43LS (solid black lines) and 90% prediction intervals (5th to 95th percentiles [dashed black lines]) according to CIS43LS dose group. Values were calculated on the basis of Monte Carlo simulations with the use of a population pharmacokinetic model. The dose groups reflect administration of CIS43LS at 5 mg per kilogram of body weight (Panel A), 20 mg per kilogram (Panel B), and 40 mg per kilogram (Panel C). Observed CIS43LS concentrations (normalized for each of the respective doses) are overlayed for comparison (circles). Panel D shows the predicted median CIS43LS concentration after a hypothetical intravenous dose of 10 mg per kilogram (dashed gray line) as compared with those of the other doses used in the trial (solid lines).
EFFICACY
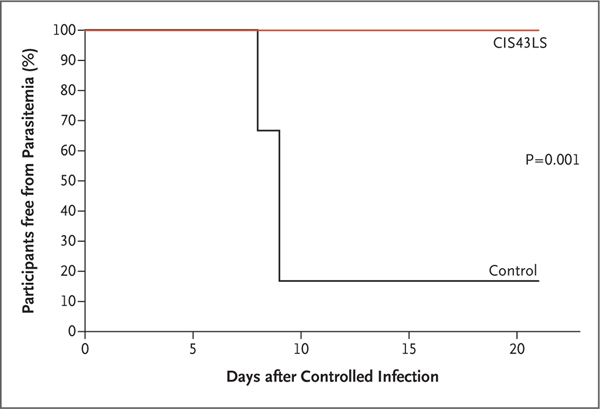
None of the 9 participants who underwent controlled human malaria infection and had received CIS43LS had parasitemia through day 21, whereas parasitemia developed in 5 of 6 control participants on days 8 or 9 after infection (P = 0.001 by two-sided Barnard test), a finding consistent with historical data for control participants who underwent infection through this model27,28 (Fig. 4). All participants who underwent controlled infection met prespecified malaria exposure criteria at the time of the challenge, which consisted of five qualifying bites from mosquitoes with a salivary gland score of 2 or greater (scores range from 0 to 4, with higher scores indicating more microscopically observed sporozoites). The median salivary gland score was 3.2 (interquartile range, 2.6 to 3.2) in mosquitoes that bit participants who had received CIS43LS and 3.1 (interquartile range, 3.0 to 3.4) in mosquitoes that bit control participants (Table S3). At the time of controlled infection, the serum concentrations of CIS43LS ranged from approximately 50 to 500 μg per milliliter among the 9 participants who had received CIS43LS. Two participants who underwent controlled infection up to 36 weeks after administration of CIS43LS had serum concentrations of approximately 50 μg per milliliter at the time of infection (Fig. 2B and Table S4).
Figure 4. Parasitemia after Controlled Human Malaria Infection.
A Kaplan–Meier analysis shows the time to parasitemia as measured by polymerase-chain-reaction analysis. A log-rank test comparing parasitemia among the nine participants who received CIS43LS with that among the six control participants yielded a P value of 0.001.
DISCUSSION
Ending the morbidity, mortality, and economic burden caused by malaria requires additional interventions. Passive administration of a potent monoclonal antibody that has a long half-life offers a new approach to the prevention of infection with a single administration, depending on the length of time protection is required. Monoclonal antibodies have already been approved or authorized for the prevention or treatment of several viral infections caused by respiratory syncytial virus, Ebola virus, and severe acute respiratory syndrome coronavirus 2.29–31 A clinical study performed more than 50 years ago showed that passive administration of gamma globulin from malaria-immune adults to persons with ongoing blood-stage infection lowered the blood-stage parasitemia in these persons.32
Here, we present evidence in humans that malaria can be prevented from 4 to 36 weeks after a single administration of a monoclonal antibody against the major protein covering the surface of the infecting sporozoite.
In this small phase 1 trial, no safety concerns associated with CIS43LS were identified after one or two intravenous administrations, as well as after subcutaneous administration. There were no infusion-related reactions or dose-limiting toxic effects. Immediate postadministration protection lasting for an extended period is a beneficial feature of monoclonal antibodies in preventing malaria. Given that the half-life of CIS43LS was 56 days, longer than the average 21-day physiologic half-life of human IgG,33,34 CIS43LS displayed a pharmacokinetic profile aligned with potential clinical use across a variety of settings.
A secondary objective in this trial was to define the CIS43LS serum concentration required to mediate protection. Data from preclinical studies in mice that used chimeric mouse Plasmodium berghei parasites containing P. falciparum circumsporozoite protein showed that a CIS43LS serum concentration of approximately 400 μg per milliliter would be required for complete protection after infection through a mosquito bite.22,24,35 We planned to achieve these levels using intravenous administration and then discern the protective serum concentration in humans by performing a prespecified regression analysis in the trial population across a range of serum concentrations at the time of controlled human malaria infection. Thus, the trial was designed with the majority of participants receiving intravenous administration. We could not determine the protective serum concentration threshold, however, because none of the participants who received CIS43LS became parasitemic. Future dose de-escalation studies are necessary to delineate this protective concentration. Once known, the predictive pharmacokinetic model developed with the data from this trial could then provide the dose necessary to maintain serum concentrations above this threshold.
The controlled human infection model involving five bites from infected mosquitoes at one time has been used for more than 30 years to provide important vaccine or drug safety and protective efficacy data.27,28 These data enable field studies to be conducted in regions in which malaria is endemic, where patient characteristics and exposure conditions differ significantly from the controlled-infection model. Accordingly, another clinical trial of CIS43LS involving adults in Mali is under way to establish the safety, pharmacokinetics, clinical feasibility, and proof of principle for protective efficacy against persistent exposure to diverse P. falciparum strains during the 6-month rainy season (ClinicalTrials.gov number, NCT04329104).
The limitations of this trial include its small size and the fact that only intravenous administration of CIS43LS was assessed because subcutaneous administration could not be evaluated. It was intended that participants who received subcutaneous CIS43LS in Part A of the trial would undergo controlled infection, but this was postponed because of Covid-19 restrictions. Nevertheless, protection resulting from intravenous administration, even in a small number of participants, is an encouraging proof of concept that passive administration of monoclonal antibodies can prevent malaria after controlled infection. Additional trials are needed to explore the feasibility of the route of administration in variable clinical settings across distinct populations. The data generated after a short intravenous infusion may most easily translate into non-endemic clinical-use cases for travelers, military personnel, and health care workers for whom a single pretravel intravenous infusion would obviate the need for daily chemoprophylaxis and limit issues related to long-term adherence. Another major focus of future research will be to determine whether protection can be achieved by subcutaneous administration of CIS43LS or more potent second-generation monoclonal antibodies currently in clinical development.35
This trial provides two major advances in malaria prevention. First, the fact that CIS43LS targets the junctional region of the P. falciparum circumsporozoite protein supports the inclusion of this site in next-generation vaccines.36–39 Second, the trial provides a potential path forward for passive prevention against malaria. The observation that a single administration of CIS43LS provided protection against malaria could have potentially broad clinical application, including in the seasonal control of malaria in regions in which it is endemic and in elimination campaigns. Future research may reveal new routes, doses, and more potent monoclonal antibodies and may allow expanded use of monoclonal antibodies in vulnerable populations such as pregnant women and children in areas where malaria is endemic.
Supplementary Material
Acknowledgments
The content of this article has been reviewed by the Walter Reed Army Institute of Research, which had no objection to its presentation or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for the protection of human participants as prescribed in Army Regulation 70-25. Drs. Kisalu, Idris, and Seder and Ms. Flynn are listed as inventors on a patent describing CIS43 and related antibodies.
Supported by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Dr. Evans’ work is funded by a grant (F0642_20_WR_CS) from the Military Infectious Disease Research Program (Malaria Vaccine Research) for maintenance of mosquito and parasite capabilities at Department of Defense laboratories worldwide.
We thank the trial participants for their commitment to malaria research and staff members of the Emmes Corporation (Phyllis Zaia Renehan, Jeanine M. May, Eugeania Burch, Kaitlyn M. Paster, Meghan K. Kunchai, and Taylor J. Rowley), staff members of Technical Resources International (Neta Nelson, Jariss Smith, and Nurat Quadri), and staff members of the United States Army Medical Research and Development Command, Office of Research Protections, Human Research Protections Office, for their contributions to this trial.
Footnotes
The members of the VRC 612 Study Team are listed in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
REFERENCES
- 1.World malaria report 2018. Geneva: World Health Organization, 2018. (http://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf). [Google Scholar]
- 2.World malaria report 2015. Geneva: World Health Organization, 2015. (http://apps.who.int/iris/bitstream/handle/10665/200018/9789241565158_eng.pdf). [Google Scholar]
- 3.The RTS S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011;365: 1863–75. [DOI] [PubMed] [Google Scholar]
- 4.RTS S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 2009; 200: 337–46. [DOI] [PubMed] [Google Scholar]
- 6.Olotu A, Lusingu J, Leach A, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis 2011; 11: 102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macintyre F, Ramachandruni H, Burrows JN, et al. Injectable anti-malarials revisited: discovery and development of new agents to protect against malaria. Malar J 2018; 17: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockburn IA, Seder RA. Malaria prevention: from immunological concepts to effective vaccines and protective antibodies. Nat Immunol 2018; 19: 1199–211. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 1980; 207:71–3. [DOI] [PubMed] [Google Scholar]
- 10.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol 2004; 34: 991–6. [DOI] [PubMed] [Google Scholar]
- 11.Sinnis P, Coppi A. A long and winding road: the Plasmodium sporozoite’s journey in the mammalian host. Parasitol Int 2007; 56: 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nardin EH, Nussenzweig V, Nussenzweig RS, et al. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med 1982;156: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med 1980; 151: 1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollingdale MR, Zavala F, Nussenzweig RS, Nussenzweig V. Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J Immunol 1982; 128: 1929–30. [PubMed] [Google Scholar]
- 15.Cerami C, Frevert U, Sinnis P, et al. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 1992; 70: 1021–33. [DOI] [PubMed] [Google Scholar]
- 16.Tewari R, Spaccapelo R, Bistoni F, Holder AA, Crisanti A. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J Biol Chem 2002; 277: 47613–8. [DOI] [PubMed] [Google Scholar]
- 17.Zavala F, Tam JP, Hollingdale MR, et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 1985; 228: 1436–40. [DOI] [PubMed] [Google Scholar]
- 18.Zavala F, Cochrane AH, Nardin EH, Nussenzweig RS, Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med 1983;157: 1947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foquet L, Hermsen CC, van Gemert G-J, et al. Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J Clin Invest 2014; 124: 140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyen D, Torres JL, Wille-Reece U, et al. Structural basis for antibody recognition of the NANP repeats in Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci U S A 2017; 114(48): E10438–E10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imkeller K, Scally SW, Bosch A, et al. Antihomotypic affinity maturation improves human B cell responses against a repetitive epitope. Science 2018; 360: 1358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisalu NK, Idris AH, Weidle C, et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med 2018; 24:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seder RA, Chang L-J, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359–65. [DOI] [PubMed] [Google Scholar]
- 24.Kisalu NK, Pereira LD, Ernste K, et al. Enhancing durability of CIS43 monoclonal antibody by Fc mutation or AAV delivery for malaria prevention. JCI Insight 2021;6(3): e143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010; 28:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishizuka AS, Lyke KE, DeZure A, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22: 614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garver LS, Dowler M, Davidson SA. Controlled human malaria infection at the Walter Reed Army Institute of Research: the past, present, and future from an entomological perspective. US Army Med Dep J 2015; July-Sep: 16–24. [PubMed] [Google Scholar]
- 28.Spring M, Polhemus M, Ockenhouse C. Controlled human malaria infection. J Infect Dis 2014; 209:Suppl 2: S40–S45. [DOI] [PubMed] [Google Scholar]
- 29.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102: 531–7. [PubMed] [Google Scholar]
- 30.Gaudinski MR, Coates EE, Novik L, et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet 2019; 393: 889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384: 238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 1961; 192: 733–7. [DOI] [PubMed] [Google Scholar]
- 33.Kolar GR, Capra JD. Immunoglobulins: structure and function. In: Paul WE, ed. Fundamental immunology. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2003. [Google Scholar]
- 34.Booth BJ, Ramakrishnan B, Narayan K, et al. Extending human IgG half-life using structure-guided design. MAbs 2018; 10:1098–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang LT, Pereira LS, Flores-Garcia Y, et al. A potent anti-malarial human monoclonal antibody targets circumsporozoite protein minor repeats and neutralizes sporozoites in the liver. Immunity 2020; 53(4):733–744.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atcheson E, Hill AVS, Reyes-Sandoval A. A VLP for validation of the Plasmodium falciparum circumsporozoite protein junctional epitope for vaccine development. NPJ Vaccines 2021; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calvo-Calle JM, Mitchell R, Altszuler R, Othoro C, Nardin E. Identification of a neutralizing epitope within minor repeat region of Plasmodium falciparum CS protein. NPJ Vaccines 2021; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francica JR, Shi W, Chuang G-Y, et al. Design of alphavirus virus-like particles presenting circumsporozoite junctional epitopes that elicit protection against malaria. Vaccines (Basel) 2021;9: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jelínková L, Jhun H, Eaton A, Petrovsky N, Zavala F, Chackerian B. An epitopebased malaria vaccine targeting the junctional region of circumsporozoite protein. NPJ Vaccines 2021; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.