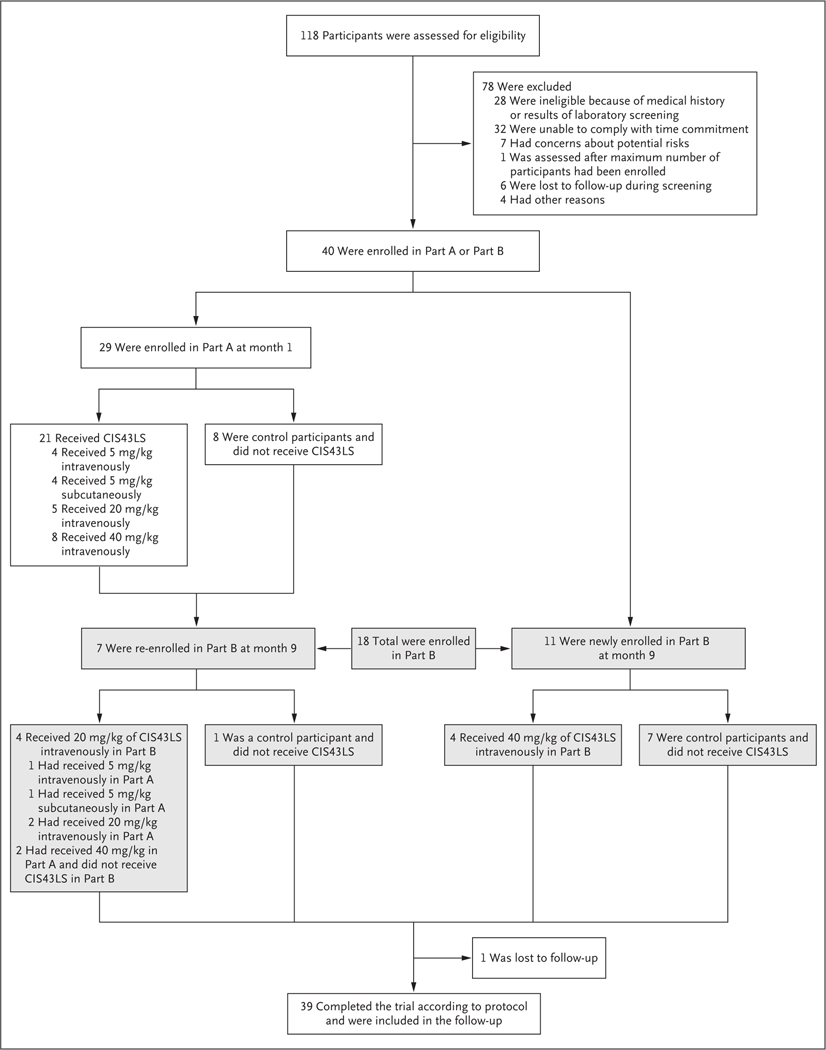
Figure 1. Participants and Administration of CIS43LS.
The VRC 612 trial was conducted in two parts. Because of restrictions related to coronavirus disease 2019, the controlled human malaria infection that was originally planned for Part A was canceled, and the trial was modified. Of the 18 participants enrolled in Part B, 15 underwent controlled infection in month 10 (October 2020): 9 of these participants had received CIS43LS and 6 were control participants.