Figure. Study Flowchart.
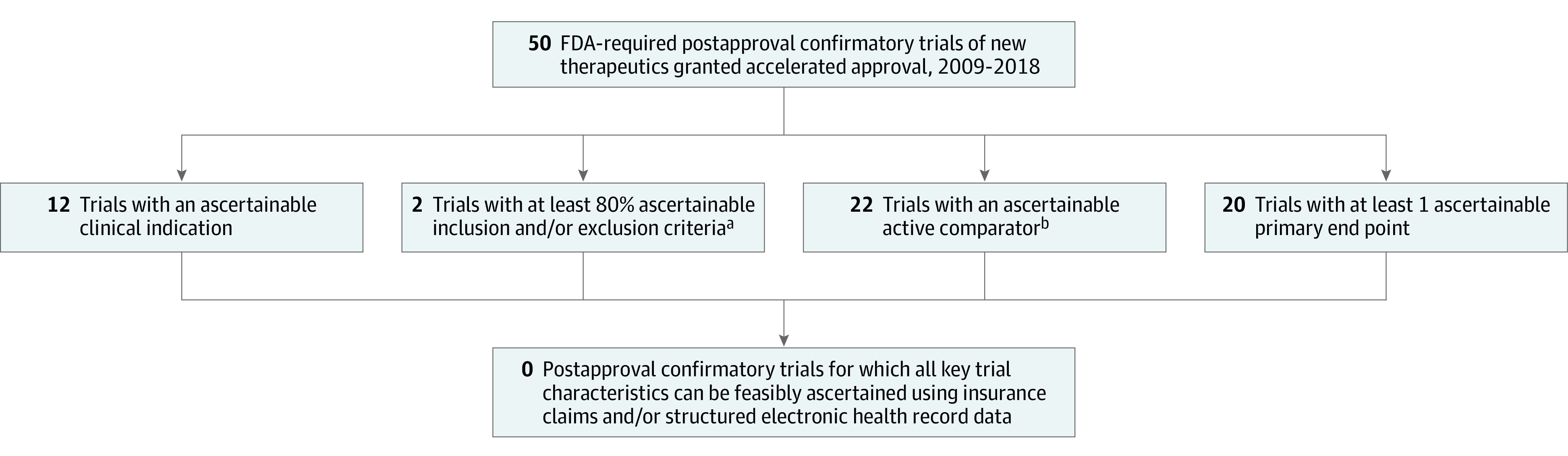
Flowchart demonstrates the feasibility of using real-world data to emulate 50 US Food and Drug Administration (FDA)–required postapproval confirmatory trials of new therapeutic agents granted accelerated approval between 2009 and 2018.
aThere were 4 trials without clearly defined inclusion and exclusion criteria (no ClinicalTrials.gov registration).
bThere were 41 trials with a comparator arm. The “active” designation includes a standard of care comparator, an active comparator, and a standard of care with an active comparator.
