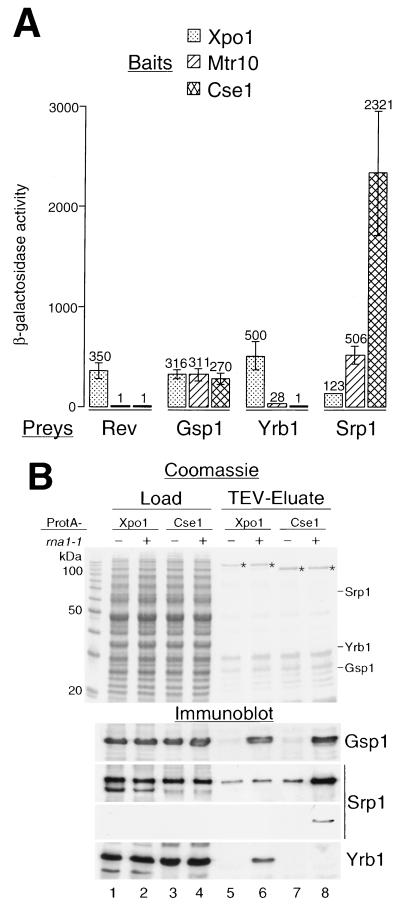
FIG. 2.
Yrb1 and Xpo1 interact in vivo. (A) Two-hybrid interaction. Full-length Yrb1 fused to the B42-TAD (Prey) was tested for interaction with full-length Xpo1, Mtr10, and Cse1 fused to the LexA DBD (Bait). B42-TAD fusion constructs of full-length HIV Rev, importin α (Srp1), and Gsp1 served as controls. β-Galactosidase activities are given in arbitrary units on top of the error bars. (B) Affinity purification of ProtA-Xpo1 from rna1-1 cells. xpo1::HIS3 cells complemented by a plasmid containing ProtA-XPO1 were grown in YPD at 23°C. As a control, cells deleted for CSE1 and rescued by ProtA-CSE1 were cultivated accordingly. The ProtA-fusion proteins were affinity purified on IgG-Sepharose and eluted using the TEV protease. The homogenate supernatants (Load) and TEV eluates were analyzed by SDS-PAGE, Coomassie blue staining, and immunoblotting using the indicated antibodies. In the case of the anti-Srp1 immunoblotting, both the enhanced chemiluminescence (upper panel) and the less-strong color reaction (4-chloro-1-naphthol, lower panel) are shown. The relative mobilities of Srp1, Yrb1, and Gsp1 in the Coomassie gel are also shown. Asterisks indicate the purified ProtA fusion proteins.