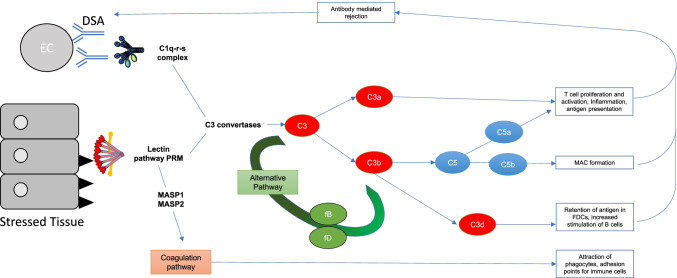
Fig. 1.
A summary of how complement is activated in IRI and transplantation alongside the downstream consequences. IRI causes stressed epithelial tissue to expose fucosylated ligands (black triangles) which are bound by lectin pathway pattern recognition molecules (PRMs). Antibody-mediated rejection (ABMR) is mediated by donor-specific antibody (DSA) which binds donor endothelial cells (ECs), at sites of inflammation—DSA is then bound by C1q, the PRM of the classical pathway. Both the classical and lectin pathway PRMs activate their respective complement pathways, ultimately producing C3 convertases that cleave C3 to C3a and C3b. C3a, amongst other roles contributes to T cell activation and proliferation. C3b causes activation of the alternative pathway which creates more C3 convertase and therefore a further increase in C3a and C3b. C3b combines with other complement pathway components to create C5 convertases and cleave C5. The resulting products of C5 cleavage are C5a which contributes to inflammation, T cell activation and proliferation and antigen presentation, as well as contributing to ABMR. Another product C5b, alongside complement components C6 to 9 form the terminal complement component, membrane attack complex (MAC). Another downstream product of C3 cleavage, C3d also contributes to retention of antigen in FDCs, and increased stimulation of B cells. Alongside the traditional complement pathway activation, the PRMs of the lectin pathway are also able to crosstalk with the coagulation pathway through the MBL-associated serine proteases (MASPs) 1 and 2 which ultimately results in the attraction of phagocytes, as well as providing adhesion points for immune cells. Epithelial and endothelial injury induced by these processes drives the cycle of complement activation and coagulation feeding back into the pathways described above