Abstract
Balamuthia mandrillaris is an opportunistic, free-living ameba that is pathogenic to humans. It has a worldwide distribution but is mainly detected in warmer regions. Balamuthia infections are rare but have been reported in both immunocompetent and immunocompromised individuals of all ages. B. mandrillaris can enter through wounds on the skin or the nose and cause cutaneous lesions and the usually fatal Balamuthia amebic encephalitis (BAE). Infection usually spreads from the lungs or through nerve fibers, and attacks the central nervous system, forming granulomatous lesions and necrosis in the brain. Balamuthia infection is usually chronic, and patients initially present with nonspecific symptoms, including headache, nausea, myalgia, and low-grade fever. As the disease progresses, the patient becomes paralyzed and comatose, often leading to death. Lack of knowledge of predisposing factors, specific treatment, and standardized detection tools have resulted in a nearly cent percent fatality rate. Although only about 200 cases have been reported worldwide since its characterization in the 1990s, the number of reported cases has increased over the years. BAE is an emerging disease and a major health concern. Few patients have survived Balamuthia infections with antimicrobial treatment that has largely been empirical. Early diagnosis is the key and requires familiarity with the disease and a high degree of suspicion on the part of the diagnostician. There are currently no specific treatment and prevention recommendations. This review highlights our current understanding of B. mandrillaris in terms of its pathogenicity, genomics, and novel diagnostic and therapeutic approaches against BAE infections.
Keywords: Balamuthia mandrillaris, free-living ameba, granulomatous amebic encephalitis, opportunistic
INTRODUCTION
Balamuthia mandrillaris is an opportunistic, free-living ameba found in soil and freshwater. It is pathogenic to humans along with other amoebozoans such as Entamoeba histolytica and several species of Acanthamoeba, Sappinia, and Endolimax. Certain species of Acanthamoeba, B. mandrillaris, Naegleria fowleri, and Sappinia can cause infections of the central nervous system (CNS) in humans and animals. Although rare, B. mandrillaris and Acanthamoeba can cause fatal granulomatous infections of the CNS known as granulomatous amebic encephalitis (GAE).
B. mandrillaris was first isolated from the brain of a pregnant mandrill monkey (Mandrillus sphinx) that died of meningoencephalitis at the San Diego Zoo Wild Animal Park in 1986[1] and was designated as a new protozoan in 1993.[2] In addition to humans, B. mandrillaris infections have been reported in other animals such as dogs, horses, and other primates.[3,4,5,6] Before the discovery of B. mandrillaris, Acanthamoeba had been regarded as the prime causal organism of GAE in humans. After the discovery, however, retrospective analysis of GAE cases from 1974 to 1989,[7] suspected to be caused by an unknown ameba, confirmed that B. mandrillaris also causes GAE in humans, known as Balamuthia amebic encephalitis (BAE).
B. mandrillaris exists in two forms in nature: cyst and trophozoite, both of which can infect humans either through the respiratory tract or open wounds in the skin. The incubation period is uncertain, ranging from weeks to years.[8,9] Following dissemination through blood, the ameba lodges itself in different organs but most commonly the brain and the skin giving rise to clinical manifestations of BAE and cutaneous balamuthiasis. It ingests pieces of host tissue, produces tissue degrading enzymes, and elicits an immune response resulting in necrosis, granuloma, hemorrhage, and lesions. Symptoms often include headache, difficulty swallowing, focal paralysis, seizures leading to coma, and eventual death in the progressive stages.[10] Infections have been reported in both immunocompetent and immunocompromised individuals of all ages[7] and over 200 cases have been reported worldwide.[11]
It is difficult to distinguish B. mandrillaris from Acanthamoeba based on light microscopic evaluation alone. It can, however, be differentiated by ultramicroscopic details, molecular diagnosis, and its inability to grow in xenic cultures (enteric bacteria-laced agar medium).[2,12] Difficulty in diagnosis, compounded by misdiagnosis,[13] insidious nature of the infection, and the lack of specific drugs have resulted in a fatality rate of nearly 100%. With an early diagnosis, in part due to disease awareness and the use of modern molecular diagnostic techniques, few have reported recovery after combination therapy of antimicrobials, albeit with severe neurologic deficits.[14]
This review discusses the current knowledge of disease characteristics and progress of therapeutic research while highlighting major challenges facing this rare but extremely fatal disease.
HISTORY
Free-living ameba causing disease in animals, including man, was first proposed by C. G. Culbertson in 1958.[15] He observed that an ameba, designated as Acanthamoeba, which was found as a contaminant in monkey kidney cell cultures during the development of a polio vaccine, when injected in mice and monkeys, resulted in encephalitis and eventual death. Culbertson, for the first time, proposed that this ameba could also infect humans.[10,16] In 1966, Carter reported another free-living ameba, N. fowleri, causing human meningoencephalitis.[17] In the years that followed, several reports of amebic meningoencephalitis in humans were reported.[18,19,20,21,22,23,24]
In 1986, a 3-year-old pregnant mandrill monkey (M. sphinx) died at the San Diego Zoo Wild Animal Park after showing signs of severe paralysis. Ameba isolated from the brain was found to be microscopically different from other known meningoencephalitis causing amebae. Unlike Acanthamoeba and Naegleria, it failed to grow in agar plates covered with Escherichia coli lawn but grew successfully in monkey kidney cells (Vero E6). In addition, the isolated ameba, along with the amebae from earlier undiagnosed cases of meningoencephalitis, did not react with the antisera made against Acanthamoeba, Naegleria, and E. histolytica.[1] This differentiated the newly identified ameba from known causative agents of amebic encephalitis in humans and was initially designated as a leptomyxid ameba. Subsequently, the ameba came to be known as B. mandrillaris, named by Govinda S. Visvesvara in honor of his late mentor, William Balamuth, and the animal it was first isolated from.[2] Ribosomal RNA (rRNA) sequencing and phylogenetic analyses have confirmed that while being closely related to Acanthamoeba, it is a distinct genus and species.[25,26] It is the only known species of Balamuthia.
Nearly 200 cases of B. mandrillaris infections have been reported worldwide, with very few survivors, even with treatment.[12] Delay in diagnosis, misdiagnosis, lack of effective drugs, and the severity of infection have been some of the contributing factors to such a low survival rate. Some cases of GAE due to B. mandrillaris present an antecedent skin lesion and hence increase the chance of an early diagnosis before the CNS involvement.[12]
EPIDEMIOLOGY
Infection of B. mandrillaris has been recognized as an emerging disease, causing severe BAE, often with skin lesions (cutaneous balamuthiasis). Exposure to soil, like playing with dirt or gardening, and inhalation of cysts carried by the wind, are likely sources of infection. In addition, a probable mode of infection can be through organ transplants.[27] Two such cases had been reported to the US Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, in 2009[28] and 2010.[29] Occasionally, B. mandrillaris can harbor bacteria such as Legionella pneumophila,[30] the causative agent of Legionnaires' disease, and act as a transmission vector. The ability of the amebae to host such pathogens can enhance the infectivity of the bacteria, which in turn can increase the pathogenicity of the ameba itself.[31]
Of the nearly 200 global cases of balamuthiasis diagnosed with brain involvement, very few have survived.[9,32] While reports over the years have indicated BAE in immunocompetent and seemingly healthy individuals, immunocompromised patients receiving immunosuppressive drugs or with HIV/AIDS, cancer, diabetes, and liver disease are at greater risk.[33] Infections appear to be higher in patients of Hispanic origin,[34] and specific age groups (under 15 years and above 60 years), likely due to their weaker immune systems.[31] A majority of cases have been reported from warmer regions globally,[35] especially Latin America (Mexico, Peru, Venezuela, Argentina, Brazil, and Chile) and the southwestern United States (mainly Arizona, Texas, and California). Few cases have been reported from the UK, Portugal, and the Czech Republic.[36] From the Asia-Pacific, cases have been reported from China, Japan, India, Thailand, South Korea, and Australia.[9] In a recent study conducted in China, out of 28 patients identified with Balamuthia infection, only 12 had survived. However, among the survivors, only 1 patient had BAE, while the rest presented clinical symptoms of cutaneous balamuthiasis.[9] It has been observed that Balamuthia reports from China and Peru generally have a higher percentage of skin infection preceding brain involvement, whereas it is rare in cases from the US.
Only a few cases have been reported worldwide each year since the identification of B. mandrillaris as the causal organism of GAE and skin infection. This low incidence rate may be, in part, due to misdiagnosis compounded by lack of disease awareness, the severity of the disease, and no specific treatment regimens. The observed low occurrence could also indicate some predisposing factor (s). Whether this factor is another underlying disease, genetic, climate, exposure to an environment with possible B. mandrillaris contamination, or a combination thereof, is unclear.[31] Interestingly, no cases have been reported from Africa, although a study from the Ivory Coast indicated high B. mandrillaris seroprevalence in healthy individuals without any symptoms.[37] This might indicate that not all Balamuthia infections are lethal and may depend on the pathogen load, pathogenicity, and the immune status of the individual. While the number of cases reported each year is fairly low, the numbers have steadily increased in the last several years. This could be due to the increasing recognition of Balamuthia infection once it was established as a human pathogen and not necessarily a true increase in the number of cases.[7]
GENOMICS
The first draft genome of B. mandrillaris from the original 1990 mandrill baboon isolate (CDC-V039) was reported by Detering et al. in 2015.[38] Next-generation sequencing (NGS) resulted in a genome size of 68 Mbp in 1605 contigs, with an N50 value of 93,953 bp and an average GC content of 46.8%. Functional annotation of the genome is currently underway. In the same year, Greninger et al. reported the first complete sequence of the mitochondrial genome of B. mandrillaris (strain 2046).[39] The 41.6 kb circular mitochondrial genome was found to have two rRNA genes, 18 transfer RNA genes, and 38 coding sequences. Subsequent sequencing of 10 other strains of B. mandrillaris has indicated mitochondrial genome sizes between 39.8–42.8 kb with 33 protein-coding genes and up to 14 tRNA genes.[40] Several strains have been identified based on differences in the whole genome, mitochondrial, 16S rRNA, 18S rRNA, RNase P gene sequences, as summarized in Table 1. Comparison of mitochondrial structure and homology has revealed a more distant relationship of B. mandrillaris to its closest known relative Acanthamoeba castellanii than was previously assumed.[38]
Table 1.
Different strains of B. mandrillaris from different sources identified based on differences in the whole genome, mitochondrial, 16S rRNA, 18S rRNA, and RNase P gene sequences
| Strain | Source | Year | GenBank Accession no. |
|---|---|---|---|
| V039 | Type strain isolated from 3-year, 10-month-old pregnant mandrill at San Diego Zoo Wild Animal Park, California, USA, cultured on Vero cells | First isolated – 1990 [1] | LFUI00000000 - WG |
| Whole-genome seq. – 2015 [38] | KT175741 - MT | ||
| MT genome seq. – 2015 [39] | |||
| V433 | 20-year-old gelding | First isolated – 1998 [5] | AF477017 |
| 16S rRNA partial seq. – 2003 [91] | |||
| SAM | 3-year-old human female from California, USA, isolated from brain, cultured on Vero cells | First isolated – Spring 2001 [92] | KT030673 |
| MT genome seq. – 2015 [39] | |||
| RP5 | Environmental sample from California, USA, associated with SAM, cultured on Vero cells | First isolated – Spring 2001 [49] | KT030672 |
| MT genome seq. – 2015 [39] | |||
| OK1 | Environmental sample in Northern California, USA, unrelated to SAM, cultured on Vero cells | First isolated – 2002 [50] | KT030671 |
| MT genome seq. – 2015 [39] | |||
| 2046 | 26-year-old human male from California, USA, survivor, cultured on Vero cells | First isolated – March 2010 [39] | LEOU00000000 - WG |
| Whole-genome seq. – 2015 [39] | KT175740 - MT | ||
| MT genome seq. – 2015 [39] | |||
| V416 | 10-year-old human female from South Brisbane, Australia, isolated from brain | Partial 16S rRNA seq. – 2003 [91] | AF477015 |
| V194 | 60-year-old human male from Las Vegas, Nevada, USA, isolated from brain | Partial 16S rRNA seq. – 2003 [91] | AF477014 |
| V188 | 60-year-old human male from Georgia, USA, isolated from brain | First isolated – 1990 [1] | KT175738 |
| MT genome seq. – 2015 [39] | |||
| GAM-19 | V188-frozen stock | MT genome seq. – 2015 [39] | KT175739 |
| AHB | Environmental sample (soil) from Northern Japan, cultured in liquid BM-3 | 16S rRNA partial seq. – 2017 [93] | LC348995 |
| V630 | An 11-year old human male of Hispanic origin from Texas, isolated from brain and CSF | 16S rRNA partial seq. – 2015 [80] | JX524851 – 16S rRNA |
| 18S rRNA partial seq. – 2015 [80] | JX524850 – 18S rRNA | ||
| Itson-1 | Environmental sample (water) | 18S rRNA partial seq. – 2014 [94] | KF874819 |
| Itson-2S | Environmental sample (soil) | 16S rRNA partial seq. – 2014 [94] | KF896587 |
| Itson-3W to Itson-9W | Environmental sample (water) | 16S rRNA partial seq. – 2014 [94] | KF896588 to KF896594 |
| PGIBAL2 | An 18-year old human male from India, isolated from brain | 16S rRNA partial seq – 2015 [95] | KF246746 |
WG – whole genome, MT – mitochondrial, rRNA – ribosomal RNA, seq. – sequenced
MORPHOLOGY
B. mandrillaris, like Acanthamoeba, has only two stages in its life cycle vegetative trophozoite and dormant cyst. The trophozoites are pleomorphic as confirmed by atomic force microscopy[41] and are 12–60 μm in diameter.[10] They are larger than Acanthamoeba trophozoites and contain a single large nucleus. Binucleate forms are commonly observed in infected tissue specimens. The nucleolus is fairly large, dense, and centrally located. Occasionally, the presence of two or more nucleoli, observed in the nucleus during an infection, differentiates it from Acanthamoeba. The trophozoites contain abundant mitochondria, ribosomes, endoplasmic reticulum, vacuoles, and form pseudopodia and filamentous structures.[42] The trophozoites are nonflagellated with multiple pseudopodia allowing them to move in a crab-like manner at speed much slower than other amebae.[43] The cysts are uninucleate and circular in shape with a mean diameter of 20 μm and a mean height of 1.46 μm.[41,44] Under a light microscope, they appear to be double-walled. However, electron microscopy reveals three distinct layers: a thin wavy ectocyst, a fibrous mesocyst, and a thick round endocyst. Morphological characteristics are shown in Figure 1. Carbohydrate analysis of the cyst wall has indicated the presence of branched glucose units, possibly as cellulose or some unidentified oligo- or polysaccharides.[45] It has been shown that ketoconazole inhibits encystment, indicating the presence of ergosterol in the membrane.[46,47] The cysts are highly resistant to repeated freeze-thawing, temperatures up to 70°C, detergents, ultraviolet radiation, chlorine, and pentamidine isethionate.[48] This resilience perhaps aids the survival of the protozoa in harsh environments for prolonged periods, leading to higher recurrence and increased pathogenicity.
Figure 1.
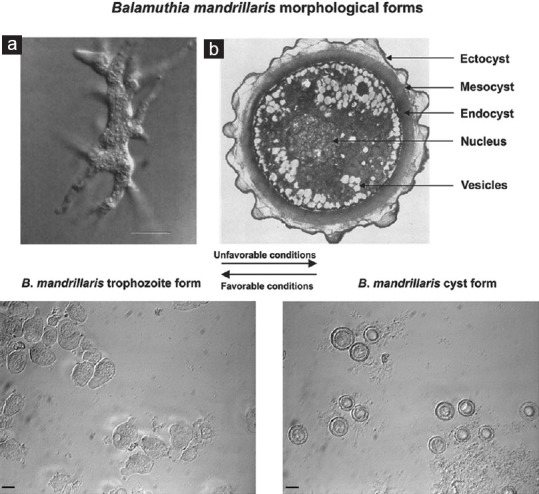
Morphological forms of Balamuthia mandrillaris. Above – (a) Interference-contrast light micrograph of a trophozoite in culture. Numerous pseudopods radiate from the ameba which helps in locomotion. Bar indicates 5 μm (Courtesy – Ref. 2). (b) Transmission electron micrograph of the cyst reveals a thin, wavy ectocyst, a fibrous mesocyst, and a thick, round, electron-dense endocyst. Magnification, ×7500 (Courtesy – Ref. 1). Below – When observed under a phase-contrast microscope, two distinct forms of the ameba can be observed. Under unfavorable conditions, the trophozoites change to cysts that appear as double-walled, circular structures under optical microscopy with a distinct nucleus and one or more nucleoli. On returning the conditions to normal, the cysts revert to the trophozoite forms. Bars indicate 10 μm (Courtesy – Ref. 31)
LIFE CYCLE
The evidence of cysts in animal samples led to the belief that B. mandrillaris was free-living. Although it had not been isolated from the environment initially, several reports recently have identified the opportunistic protozoan in soil and water samples.[49,50,51] While the environmental presence of B. mandrillaris has been confirmed, its exact ecological niche and feeding habit largely remains unknown. It is an opportunistic pathogen that lives freely in soil, water, and air but can occasionally be pathogenic to humans. The trophozoites are vegetative and divide asexually through binary fission. The cysts are dormant and are highly resilient. Both the trophozoite and the cyst can infect humans, either through breaks on the skin or through the lungs after entering the nose [Figure 2]. Following hematogenous spread from the lower respiratory tract, it can occupy host organs, specifically the brain, where it causes GAE and eats away brain tissue by phagocytosis.[31] The pathogen may also disseminate to the skin, causing lesions known as cutaneous balamuthiasis. Skin infection often precedes CNS involvement. The resilient nature of the cyst wall protects the ameba from harsh environments, which may facilitate disease transmission and also resist chemotherapy, leading to recurrent infections.[36]
Figure 2.
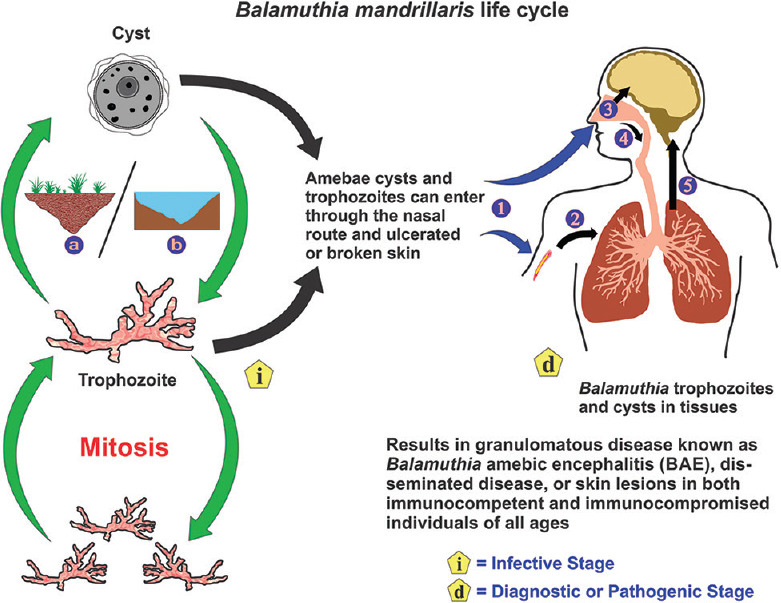
The life cycle of Balamuthia mandrillaris. Balamuthia mandrillaris is an opportunistic pathogen that is found in soil (a) and freshwater (b) that can occasionally infect humans. It exists either as dormant cysts or vegetative trophozoites that divide asexually through mitosis. The trophozoites and cysts can gain entry into the body either through a cutaneous wound or nasal passages (1). On entry through the skin (2), the amebae can reach the lungs through the blood. On entry through the nasal passages, the amebae can enter either the CNS through migration along the olfactory neuroepithelium (3) or migrate to the lower respiratory tract (4). Following hematogenous spread from the lungs, the amebae can invade the central nervous system (5) by crossing the blood–brain barrier causing granulomatous amebic encephalitis or spread to other tissues causing disseminated disease, or skin lesions
PATHOGENESIS
B. mandrillaris can enter the human body through an open wound, the respiratory tract, and on rare occasions, through organ transplants. In blood, the ameba successfully evades host immune responses, likely through the action of ecto-ATPases expressed on its surface that cleaves adenosine triphosphate released by activated cytotoxic T-cells.[31] From the lungs, the ameba disseminates to other organs through the blood. However, migration along nerve fibers has also been proposed.[36] In most cases of Balamuthia infection, two organs are primarily affected: the brain and the skin, causing BAE and cutaneous balamuthiasis, respectively. To cause BAE, the ameba has to cross the selectively permeable blood–brain barrier (BBB). Using primary human brain microvascular endothelial cells (HBMECs), it has been shown that the ameba can induce the production of the pleiotropic cytokine interleukin-6 (IL-6) from HBMECs through its activation of phosphatidylinositol 3-kinase and serine/threonine protein kinase, Akt.[52] IL-6 helps the ameba breach the BBB by increasing its permeability through modulation of adhesion molecule expression[53] and also initiates the early inflammatory response.[52]
B. mandrillaris can traverse the BBB in three ways. The first is through contact-dependent receptor-mediated transport involving adhesion to the endothelium via adhesins. Balamuthia interacts with host extracellular matrix proteins such as collagen-1, fibronectin, and laminin-1.[54] The ameba expresses a galactose-binding protein on its surface,[54,55,56] which may participate in recognition of lamin. The second is through a mechanism whereby the ameba passes in between the endothelial cells at the tight junctions, probably through its interactions with zonula and occludin proteins.[31] The third and most likely is through damage and penetration of the endothelium. A combination of adhesion, cellular damage, and inflammatory response can disrupt the BBB. Once the ameba breaches the BBB, efficient killing of the host cells through phagocytosis and induction of apoptosis ensues. The gregarious trophozoites appear both polymorphic and smooth circular in shape. Both these forms can bind multiple host cells at a time and begin to engulf and/or penetrate the target cells. In addition, Balamuthia produces metalloproteinases,[57] phospholipase A2, and phospholipase D[58] which may aid in the destruction and migration through host tissue.
Cutaneous balamuthiasis, in some cases, precedes BAE, especially in Peruvian patients.[43] Skin infections are presented as painless lesions, mostly on the face, but also on the trunk and limbs.[59] Skin biopsy shows tuberculoid granulomas in the dermis and hypodermis but rarely in the epidermis. While leukocytic infiltrates and vasculitis are common, necrosis is fairly rare. Infiltrates tend to be perineural in distribution. Cutaneous balamuthiasis serves as a strong indicator of CNS involvement.
CLINICAL MANIFESTATIONS
Disease due to B. mandrillaris is similar to that of Acanthamoeba in terms of disease progression and pathology. The amebae cause hemorrhagic necrosis in the cerebrum, midbrain, thalamus, brain stem, and cerebellum or painless pruritic lesions on the skin. Infection due to Balamuthia can be differentiated from that of Acanthamoeba in that Balamuthia can also infect both the young and the old. On contraction of Balamuthia infection, the first clinical symptoms include headache and photophobia, often followed by hemiparesis, stiff neck, myalgia, nausea, and low-grade fever. Weight loss, behavioral changes, speech defects, and focal seizures soon follow as the disease progresses to CNS involvement leading to paralysis, coma, and ultimately, death. Several cases, especially from Peru, have presented with one or multiple skin lesions in the form of erythematous plaques of a few millimeters thick and several millimeters wide. Skin infections with Balamuthia almost always progress to BAE except in few reported cases, for example, in a case from Peru, the skin infection did not progress to the CNS stage, even after 8 years.[43] These painless nodular lesions are mostly found on the face with occasional infections of the sinus cavities. However, skin infections on the trunk, hands, and feet have also been observed. The period between the appearance of skin lesions and CNS involvement may range from a few weeks to several years. Skin infections might indicate a site of entry or hematogenous spread from the lungs as amebae have also been found in kidneys, thyroid, adrenal glands, and pancreas.[31] The presence of Balamuthia trophozoites and cysts in biopsies of the skin and the brain are confirmatory for balamuthiasis.
LABORATORY DIAGNOSIS
Symptoms of B. mandrillaris infection are often subtle and nonspecific and therefore pose a significant challenge to timely diagnosis. The rarity of the disease compounded by its severity often results in diagnosis too late for specific medical intervention and is usually postmortem. The presence of painless pruritic skin lesions, especially on the face, aids in the diagnosis. Several methods have been developed, over the years, as summarized in Table 2, for the successful diagnosis of B. mandrillaris infection.
Table 2.
Diagnostic methods for human balamuthiasis
| Diagnostic approaches | Methods | Targets/purpose | Comments |
|---|---|---|---|
| Direct microscopy | Biopsy, lumbar puncture (fresh or preserved samples) H and E stain, Giemsa, PAS, calcofluor white | Detection of trophozoites and cysts in tissues/CSF | Can indicate a possible infection Drawback: Invasive and nonspecific, difficult to distinguish from Acanthamoeba. Can be differentiated by electron microscopy based on cyst morphology |
| Cultivation | Axenic, xenic, or tissue culture | Growth of ameba | Can be used to distinguish from Acanthamoeba since B. mandrillaris does not grow as xenic cultures |
| Immunodiagnostics | IHC, immunocytochemistry, IIF, ELISA, flow cytometry | Detection of anti-B. mandrillaris antibodies, trophozoites, and cysts in biopsy tissue samples, CSF, and plasma | Confirms active infection. Does not cross-react with other FLA Limitation: Uninfected individuals may also present a high titer of anti-Balamuthia antibodies |
| Molecular assays | Conventional PCR, real-time PCR (SYBR green or TaqMan) | 18S rRNA, 16S rRNA, and RNase P genes | Rapid, confirmatory, high sensitivity and specificity Limitation: Requires skilled personnel |
| Neuroimaging | CT MRI | Lesions, edema, hemorrhage | Detection of ring-enhancing lesions confirms active infection Limitation: Nonspecific |
| Metagenomics | NGS of cell-free DNA in CSF, plasma, or biopsy samples | 18S rRNA, 16S rRNA, and RNase P genes | Confirmatory, high sensitivity and specificity Limitations: Requires skilled personnel, time-consuming, requires extensive bioinformatics knowledge |
| MS | MALDI-TOF MS | 2-14 kDa proteins in whole spores or lysates | B. mandrillaris specific 2-14 kDa can distinguish between other pathogenic FLA |
CSF: Cerebrospinal fluid, B. mandrillaris: Balamuthia mandrillaris, IHC: Immunohistochemistry, IIF: Indirect immunofluorescence, PCR: Polymerase chain reaction, FLA: Free-living amebae, rRNA: Ribosomal RNA, CT: Computed tomogram, MRI: Magnetic resonance imaging, NGS: Next-generation sequencing, MALDI: Matrix-assisted laser desorption/ionization, TOF: Time of flight, MS: Mass spectrometry, PAS: Periodic acid-Schiff, H and E: Hematoxylin and eosin
Microscopy
Biopsies of skin lesions, sinuses, lungs, and the brain have been the samples of choice for detection of B. mandrillaris infection. Formalin-fixed paraffinized biopsy specimens stained with a variety of stains such as hematoxylin and eosin, Giemsa, and periodic acid–Schiff may indicate Balamuthia trophozoites scattered in the perivascular space.[60] The cysts can be visualized by calcofluor white, which binds to glycans on the cyst wall.[31] Trophozoites appear circular during infection and are often mistaken for macrophages or necrosed keratinocytes.[12] Trophozoites are rare in cerebrospinal fluid (CSF) wet mounts. However, concentrated samples may reveal the pleomorphic trophozoites with multiple pseudopodia and sluggish ameboid movement. The absence of flagella and the larger size can differentiate it from N. fowleri and Acanthamoeba spp., respectively.[12] The cysts can only be differentiated by electron microscopy based on the triple-walled cyst of B. mandrillaris compared to the bilayer cyst of Acanthamoeba.[2] The nuclei of B. mandrillaris often have two or three densely staining nucleoli as opposed to only one in Acanthamoeba, N. fowleri, and Sappinia diploidea.[43] Granulomas with CD4 and CD8 T-cells, B-lymphocytes, few plasma cells, macrophages, and multinucleated giant cells are often observed.[33,61] B. mandrillaris can be identified by indirect immunofluorescence (IIF) staining of biopsy samples with anti-Balamuthia sera.[62] B. mandrillaris can also be identified in formalin-fixed paraffinized biopsy specimens using immunohistochemistry with polyclonal rabbit anti-B. mandrillaris antibodies. The location of positive reaction, parasite staining pattern, and fluorescence intensity can aid in the interpretation of the results.[63] Microscopic characteristics to diagnose Balamuthia are depicted in Figure 3.
Figure 3.
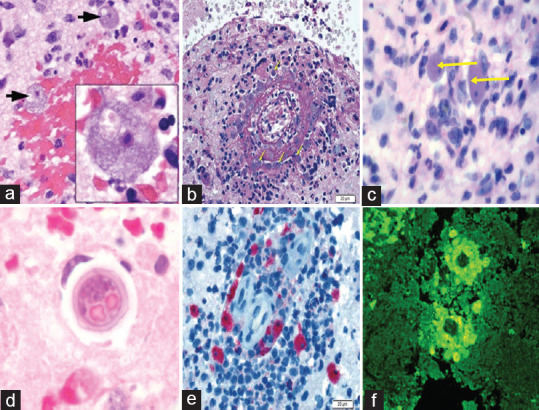
Microscopic laboratory diagnosis of Balamuthia mandrillaris. (a ) ×20 and ×100 fields of view (inset) of a brain biopsy specimen from a patient demonstrating numerously viable, large amebae (black arrows), with abundant basophilic vacuolated cytoplasm, round central nuclei, and prominent nucleoli, consistent with Balamuthia mandrillaris. Areas of extensive hemorrhagic necrosis accompanied by a polymorphic inflammatory cell infiltrate including neutrophils and eosinophils are observed. (b) Periodic acid–Schiff-stained section of brain parenchyma shows necrotizing vasculitis with mixed inflammation including macrophages and numerous eosinophils and a population of amebic trophozoites that closely resemble macrophages (yellow arrows), bar = 20 μm. (c) Typical trophozoites (H and E) under high power (×1000) are indicated by yellow arrows. (d) High-power photomicrograph of a cyst (H and E) in brain tissue. (e) Immunohistochemistry highlights numerous B. mandrillaris trophozoites, bar = 20 μm. (f) Indirect immunofluorescence using anti-Balamuthia antibodies under ultraviolet microscopy. (Image courtesy – [a] – Ref. 39, [b and e] – Ref. 67, [c] – Ref. 33, [d and f] – Centers for Disease Control and Prevention, Atlanta, USA)
Culture
Different culturing methods can be used to differentiate B. mandrillaris from other free-living pathogenic amebae. Unlike Acanthamoeba and N. fowleri, B. mandrillaris does not grow on bacteria-coated agar medium (xenic culture) and grows only on animal feeder cells in culture or as axenic cultures (cell-free growth media). For feeder cell culture, several cell lines such as African green monkey kidney cells (ATCC CRL 1586) also known as Vero E6,[1,47] rat glioma cell line (ATCC CCL 107),[64] human lung fibroblasts, and HBMEC[12] grown in media such as RPMI-1640 or Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics/antimycotics can support the growth of B. mandrillaris. Axenic cultures of Balamuthia require complex media such as BM-3,[47] modified Chang's special medium,[65] and modified Cerva's medium.[66] Tissue biopsy specimens (fresh or frozen) are directly added either in axenic media or feeder cell tissue cultures. Xenic cultures are also simultaneously inoculated. The presence of ameba is observed after incubating the cultures for the desired time. Since B. mandrillaris is negative for enflagellation test and does not grow in xenic media, it can be differentiated from other pathogenic amebae. CSF is rarely used for culture since B. mandrillaris trophozoites are occasionally observed.[12] B. mandrillaris is a slow-growing organism and therefore prolonged periods may be needed for their culture, thus making it not suitable as a quick diagnostic method.
Cerebrospinal fluid, vitreous fluid, and serology
CSF collected by a lumbar puncture between the third and fourth vertebrae can reveal lymphocytic pleocytosis with elevated protein (≥40 mg/dL) and normal-to-low glucose.[43] Since Balamuthia infection is a slowly progressing disease, detection of anti-B. mandrillaris antibody helps in the diagnosis and treatment. Anti-B. mandrillaris antibodies can be detected in the serum using IIF, ELISA, and flow cytometry.[67,68,69] The presence of Balamuthia ameba in the serum can also be detected by flow cytometry using polyclonal rabbit anti-B. mandrillaris antibodies.[37,68]
Neuroimaging
Computed tomography (CT) and magnetic resonance imaging (MRI) findings are generally nonspecific. CT or MRI with a contrast material indicates ring-enhancing lesions with surrounding edema that can be caused by an infection or inflammation including GAE, viral or bacterial meningoencephalitis, encephalomyelitis, toxoplasmosis, neurocysticercosis, or tumor.[9,13,36] MRI has revealed that hemorrhagic lesions can form in almost all parts of the brain. In T1-weighted imaging, they appear as hypodense regions, and in T2-weighted imaging, they appear hyperintense.[9,70]
Molecular diagnosis
Polymerase chain reaction (PCR) is a powerful tool in the diagnosis of pathogens. Although a conventional PCR may be used since quantification is not essential, real-time PCR is given preference as it reduces postamplification handling, time of processing, and sample contamination.[71,72] To reduce chances of nonspecific amplification and sample cross-reactivity, and to enable detection of different strains, more than one target region of the pathogen's genome are amplified in parallel.[73] Common targets include B. mandrillaris-specific mitochondrial 16S rRNA gene, nuclear 18S rRNA gene, and RNAse P gene.[9,25,71,73,74] Using real-time PCR with TaqMan probes, a minimum of approximately 1–2 amebae/specimen can be detected when targeting 16S rRNA and RNase P genes. The detection limit is much lower, about 0.2 ameba/specimen when targeting the 18S rRNA gene.[12,71] Multiplex and nested real-time PCRs can detect B. mandrillaris in CSF, vitreous fluid, tissue biopsy samples, soil, water, and archived paraffinized tissue samples.[36] The CDC uses a multiplex PCR for diagnosis of pathogenic ameba targeting 18S rRNA gene sequences employing specific primers and TaqMan probes for simultaneous detection of Acanthamoeba, N. fowleri, and B. mandrillaris.[71]
Another definitive and time-saving diagnostic tool is NGS.[75,76] Cell-free DNA can be extracted from samples such as CSF and plasma. Using metagenomic NGS and SURPI bioinformatics analysis, raw sequence data from the sample can be mapped to the available B. mandrillaris genome (draft genome published in 2015[38]) to identify the ameba.[39] Recently, several reports have described the use of NGS in confirming B. mandrillaris infection by targeting 18S rRNA and 16S rRNA genes.[39,77,78,79,80] In addition, matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry can be employed as a rapid diagnostic tool to identify characteristic protein patterns of B. mandrillaris ranging from 2 to 14 kDa.[43]
Differential diagnosis
The differential diagnosis of B. mandrillaris skin infection, particularly on the face, is with leishmaniasis, sporotrichosis, lupus vulgaris, sarcoidosis, granuloma faciale, basal cell carcinomas, natural killer and T-cell lymphomas, and Wegener's granulomatosis.[59] The differential of skin infections other than on the face is with leprosy, granuloma annulare, mycosis fungoides, and necrobiosis lipoidica. In the case of BAE, the differential diagnosis is with neurotuberculosis, neurocysticercosis, fungal infections, neoplasms, viral meningoencephalitis, and acute disseminated encephalomyelitis.[12,78]
Although several methods have been devised to effectively detect B. mandrillaris infection in humans, a high degree of suspicion on the part of the diagnostician and the familiarity of the pathologist with the amebae in tissue samples is the key to a timely diagnosis.
TREATMENT
Since Balamuthia GAE presents nonspecific symptoms, empirical treatment with steroids, antibacterials, antifungals, and antivirals has been mostly ineffective. The administration of anti-inflammatory steroids has enhanced the hematogenous spread of the amebae by depleting immune effectors.[43] There is currently no effective treatment for Balamuthia infections. Almost all reported cases had been diagnosed at late stages and therefore have resulted in a high mortality rate. Successful treatment is strongly dependent on early diagnosis when the disease may be manageable. About half the survivors of BAE had presented with characteristic skin infections and could be diagnosed early.[43,81] Recommended treatment is based on therapy provided to the small number of Balamuthia infection survivors. The treatment regimen includes surgical resection of lesions and antimicrobial combination therapy with pentamidine (4 mg/kg/day – intravenous [IV]), sulfadiazine (1.5 g every 6 h daily – oral), flucytosine (37.5 mg/kg every 6 h – oral), fluconazole (12 mg/kg/day in one dose – oral or IV), azithromycin (500 mg/day in 1 dose – oral or IV), clarithromycin (500 mg/day in 1 dose – oral), and miltefosine (2.5 mg/kg/day for pediatric cases, 100 mg/day for bodyweight <45 kg, and 150 mg/day for bodyweight ≥45 kg – oral). A recent report[82] noted the recovery of a patient with BAE with a combination therapy of azithromycin, flucytosine, and sulfadiazine and a 2010 case study has reported the successful use of fluconazole, albendazole, and oral miltefosine (150 mg per day for 12 days and 100 mg per day thereafter) for a period of 2 months.[83]
Recent studies with amlodipine, apomorphine, artemisinin, cycloheximide, cytochalasin D, demethoxycurcumin, gramicidin, haloperidol, ketoconazole, loperamide, nitroxoline, paromomycin sulfate, polymyxin B, prochlorperazine, procyclidine, and resveratrol have shown promise in vitro.[56,84,85] The traditional route of drug development is time-consuming and expensive, costing about $2.6 billion per drug to bring to the market. Hence, screening of already approved drugs as potential drug candidates against Balamuthia has proven to be both less expensive and time-consuming. Several U. S. Food and Drug Administration (FDA)-approved drugs for various ailments, including plicamycin, ponatinib, milciclib maleate, auranofin, panobinostat, bortezomib, and astemizole, have shown anti-Balamuthia activity in vitro.[86]
Most of the FDA-approved anti-Balamuthia drugs show harmful side effects compounded by their lack of target specificity and penetration, especially through the BBB. Nanoparticle conjugated drugs have been shown to circumvent these problems and can enhance the efficacy of available drug therapies.[87]
PREVENTION
Currently, there are no specific prevention protocols since it is not known why some individuals are infected while others are not. Moreover, individuals of all age groups who are both immunocompetent and immunocompromised have been infected. However, several cases have been reported in patients who have had contact with soil[49,88,89] or have been immunocompromised.[90,91,92,93,94,95] Protective clothing must be worn when working with soil or water and children must be advised against playing with soil or sand.
Transmission of Balamuthia infection from one human to another has only been reported in organ donation/transplant cases.[27,28,29]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the Sri Balaji Vidyapeeth, Deemed to be University, for facilitating the conduct of this article.
REFERENCES
- 1.Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28:2750–6. doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visvesvara GS, Schuster FL, Martinez AJ. Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and other animals. J Eukaryot Microbiol. 1993;40:504–14. doi: 10.1111/j.1550-7408.1993.tb04943.x. [DOI] [PubMed] [Google Scholar]
- 3.Hodge PJ, Kelers K, Gasser RB, Visvesvara GS, Martig S, Long SN. Another case of canine amoebic meningoencephalitis – The challenges of reaching a rapid diagnosis. Parasitol Res. 2011;108:1069–73. doi: 10.1007/s00436-010-2197-z. [DOI] [PubMed] [Google Scholar]
- 4.Rideout BA, Gardiner CH, Stalis IH, Zuba JR, Hadfield T, Visvesvara GS. Fatal infections with Balamuthia mandrillaris (a free-living amoeba) in gorillas and other Old World primates. Vet Pathol. 1997;34:15–22. doi: 10.1177/030098589703400103. [DOI] [PubMed] [Google Scholar]
- 5.Kinde H, Visvesvara GS, Barr BC, Nordhausen RW, Chiu PH. Amoebic meningoencephalitis caused by Balamuthia mandrillaris (leptomyxid amoeba) in a horse. J Vet Diagnostic Investig. 1998;10:378–81. doi: 10.1177/104063879801000416. [DOI] [PubMed] [Google Scholar]
- 6.Finnin PJ, Visvesvara GS, Campbell BE, Fry DR, Gasser RB. Multifocal Balamuthia mandrillaris infection in a dog in Australia. Parasitol Res. 2007;100:423–6. doi: 10.1007/s00436-006-0302-0. [DOI] [PubMed] [Google Scholar]
- 7.Cope JR, Landa J, Nethercut H, Collier SA, Glaser C, Moser M, et al. The epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974-2016. Clin Infect Dis. 2019;68:1815–22. doi: 10.1093/cid/ciy813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez AJ, Visvesvara GS. Balamuthia mandrillaris infection. J Med Microbiol. 2001;50:205–7. doi: 10.1099/0022-1317-50-3-205. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Cheng W, Li B, Jian Z, Qi X, Sun D, et al. Balamuthia mandrillaris infection in China: A retrospective report of 28 cases. Emerg Microbes Infect. 2020;9:2348–57. doi: 10.1080/22221751.2020.1835447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visvesvara GS. Infections with free-living amoebae. In: Garcia H, Tanowitz H, Del BO, editors. Neuroparasitology and Tropical Neurology. 3rd Ser. Vol. 114. Amsterdam: Elsevier B.V; 2013. pp. 153–68. [Google Scholar]
- 11.Takei K, Toyoshima M, Nakamura M, Sato M, Shimizu H, Inoue C, et al. An acute case of granulomatous amoebic encephalitis-Balamuthia mandrillaris infection. Intern Med. 2018;57:1313–6. doi: 10.2169/internalmedicine.0011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parija SC, Venugopal H, Dinoop K. Management of granulomatous amoebic encephalitis: Laboratory diagnosis and treatment. Trop Parasitol. 2015;5:23–8. doi: 10.4103/2229-5070.149889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piper KJ, Foster H, Susanto D, Maree CL, Thornton SD, Cobbs CS. Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage. Int J Infect Dis. 2018;77:18–22. doi: 10.1016/j.ijid.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Cary LC, Maul E, Potter C, Wong P, Nelson PT, Given C, 2nd, et al. Balamuthia mandrillaris meningoencephalitis: Survival of a pediatric patient. Pediatrics. 2010;125:699–703. doi: 10.1542/peds.2009-1797. [DOI] [PubMed] [Google Scholar]
- 15.Culbertson CG, Smith JW, Minner JR. Acanthamoeba: Observations on animal pathogenicity. Science. 1958;127:1506. doi: 10.1126/science.127.3313.1506. [DOI] [PubMed] [Google Scholar]
- 16.Culbertson CG. Pathogenic Acanthamoeba (Hartmanella) Am J Clin Pathol. 1961;35:195–202. doi: 10.1093/ajcp/35.3.195. [DOI] [PubMed] [Google Scholar]
- 17.Carter RF. Description of a Naegleria sp.isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970;100:217–44. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- 18.Fowler M, Carter RF. Acute pyogenic meningitis probably due to Acanthamoeba sp.: A preliminary report. Br Med J. 1965;2:740–2. doi: 10.1136/bmj.2.5464.734-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butt CG. Primary amebic meningoencephalitis. N Engl J Med. 1966;274:1473–6. doi: 10.1056/NEJM196606302742605. [DOI] [PubMed] [Google Scholar]
- 20.Červa L, Novak K, Culbertson CG. An outbreak of acute, fatal amoebic meningoencephalitis. Am J Epidemiol. 1968;88:436–44. doi: 10.1093/oxfordjournals.aje.a120905. [DOI] [PubMed] [Google Scholar]
- 21.Duma RJ, Ferrell HW, Nelson EC, Jones MM. Primary amebic meningoencephalitis. N Engl J Med. 1969;281:1315–23. doi: 10.1056/NEJM196912112812401. [DOI] [PubMed] [Google Scholar]
- 22.dos Santos Neto G. Fatal primary amebic meningoencephalitis.A retrospective study in Richmond, Virginia. Am J Clin Pathol. 1970;54:737–42. doi: 10.1093/ajcp/54.5.737. [DOI] [PubMed] [Google Scholar]
- 23.Lawande RV, Macfarlane JT, Weir WR, Awunor-Renner C. A case of primary amoebic meningoencephalitis in a Nigerian farmer. Am J Trop Med Hyg. 1980;29:21–5. doi: 10.4269/ajtmh.1980.29.21. [DOI] [PubMed] [Google Scholar]
- 24.Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J. Successful treatment of primary amoebic meningoencephalitis. N Engl J Med. 1982;306:346–8. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- 25.Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg. 2003;68:65–9. [PubMed] [Google Scholar]
- 26.Stothard DR, Schroeder-Diedrich JM, Awwad MH, Gast RJ, Ledee DR, Rodriguez-Zaragoza S, et al. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J Eukaryot Microbiol. 1998;45:45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basavaraju SV, Kuehnert MJ, Zaki SR, Sejvar JJ. Encephalitis caused by pathogens transmitted through organ transplants, United States, 2002-2013. Emerg Infect Dis. 2014;20:1443–51. doi: 10.3201/eid2009.131332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlessinger S, Kokko K, Fratkin J, Butt F, Hawxby A, Todaro M, et al. Balamuthia mandrillaris transmitted through organ transplantation-Mississippi, 2009. Morb Mortal Wkly Rep. 2010;59:1165–70. [PubMed] [Google Scholar]
- 29.Mbaeyi C. Transplant-transmitted Balamuthia mandrillaris-Arizona, 2010. Morb Mortal Wkly Rep. 2010;59:1182. [PubMed] [Google Scholar]
- 30.Sheba Shadrach W, Rydzewski K, Laube U, Holland G, Kiderlen AF, Flieger A. Opportunistic agent of encephalitis, is a potential host for Legionella pneumophila bacteria. Appl Environ Microbiol. 2005;71:2244–9. doi: 10.1128/AEM.71.5.2244-2249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matin A, Siddiqui R, Jayasekera S, Khan NA. Increasing importance of Balamuthia mandrillaris. Clin Microbiol Rev. 2008;21:435–48. doi: 10.1128/CMR.00056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollmer ME, Glaser C. A Balamuthia survivor. JMM Case Rep. 2016;3:e005031. doi: 10.1099/jmmcr.0.005031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yohannan B, Feldman M. Case report fatal Balamuthia mandrillaris encephalitis. Case Rep Infect Dis. 2019;2019:1–5. doi: 10.1155/2019/9315756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster FL, Glaser C, Honarmand S, Maguire JH, Visvesvara GS. Balamuthia amoebic encephalitis risk, Hispanic-Americans. Emerg Infect Dis. 2004;10:1510–2. doi: 10.3201/eid1008.040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34:1001–27. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzo-Morales J, Cabello-Vílchez AM, Martín-Navarro CM, Martínez-Carretero E, Piñero JE, Valladares B. Is Balamuthia mandrillaris a public health concern worldwide? Trends Parasitol. 2013;29:483–8. doi: 10.1016/j.pt.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Kiderlen AF, Radam E, Schuster FL, Adjogoua EV, Akoua-Koffi C, Leendertz FH. Balamuthia and Acanthamoeba-binding antibodies in West African human sera. Exp Parasitol. 2010;126:28–32. doi: 10.1016/j.exppara.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Detering H, Aebischer T, Dabrowski PW, Radonić A, Nitsche A, Renard BY, et al. First draft genome sequence of Balamuthia mandrillaris, the causative agent of amoebic encephalitis. Genome Announc. 2015;3:10–1. doi: 10.1128/genomeA.01013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greninger AL, Messacar K, Dunnebacke T, Naccache SN, Federman S, Bouquet J, et al. Clinical metagenomic identification of Balamuthia mandrillaris encephalitis and assembly of the draft genome: The continuing case for reference genome sequencing. Genome Med. 2015;7:1–4. doi: 10.1186/s13073-015-0235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bondarenko N, Smirnov A, Nassonova E, Glotova A, Fiore-Donno AM. Mitochondrial genomes of amoebozoa. Protistology. 2019;13:179–91. [Google Scholar]
- 41.Aqeel Y, Siddiqui R, Ateeq M, Raza Shah M, Kulsoom H, Khan NA. Atomic force microscopic imaging of Acanthamoeba castellanii and Balamuthia mandrillaris trophozoites and cysts. J Eukaryot Microbiol. 2015;62:85–94. doi: 10.1111/jeu.12147. [DOI] [PubMed] [Google Scholar]
- 42.Jayasekera S, Sissons J, Tucker J, Rogers C, Nolder D, Warhurst D, et al. Post-mortem culture of Balamuthia mandrillaris from the brain and cerebrospinal fluid of a case of granulomatous amoebic meningoencephalitis, using human brain microvascular endothelial cells. J Med Microbiol. 2004;53:1007–12. doi: 10.1099/jmm.0.45721-0. [DOI] [PubMed] [Google Scholar]
- 43.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqui R, Khan NA. Balamuthia mandrillaris: Morphology, biology, and virulence. Trop Parasitol. 2015;5:15–22. doi: 10.4103/2229-5070.149888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siddiqui R, Khan NA, Jarroll EL. The cyst wall carbohydrate composition of Balamuthia mandrillaris. Parasitol Res. 2009;104:1439–43. doi: 10.1007/s00436-009-1346-8. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui R, Matin A, Warhurst D, Stins M, Khan NA. Effect of antimicrobial compounds on Balamuthia mandrillaris encystment and human brain microvascular endothelial cell cytopathogenicity. Antimicrob Agents Chemother. 2007;51:4471–3. doi: 10.1128/AAC.00373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuster FL, Visvesvara GS. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amoebic meningoencephalitis in humans and other animals. J Clin Microbiol. 1996;34:385–8. doi: 10.1128/jcm.34.2.385-388.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siddiqui R, Ortega-Rivas A, Khan NA. Balamuthia mandrillaris resistance to hostile conditions. J Med Microbiol. 2008;57:428–31. doi: 10.1099/jmm.0.47694-0. [DOI] [PubMed] [Google Scholar]
- 49.Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, et al. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol. 2003;41:3175–80. doi: 10.1128/JCM.41.7.3175-3180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology (Reading) 2004;150:2837–42. doi: 10.1099/mic.0.27218-0. [DOI] [PubMed] [Google Scholar]
- 51.Baquero RA, Reyes-Batlle M, Nicola GG, Martín-Navarro CM, López-Arencibia A, Guillermo Esteban J, et al. Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog Glob Health. 2014;108:206–11. doi: 10.1179/2047773214Y.0000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayasekera S, Matin A, Sissons J, Maghsood AH, Khan NA. Balamuthia mandrillaris stimulates interleukin-6 release in primary human brain microvascular endothelial cells via a phosphatidylinositol 3-kinase-dependent pathway. Microbes Infect. 2005;7:1345–51. doi: 10.1016/j.micinf.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 53.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 54.Rocha-Azevedo B, Jamerson M, Cabral GA, Silva-Filho FC, Marciano-Cabral F. The interaction between the amoeba Balamuthia mandrillaris and extracellular matrix glycoproteins in vitro. Parasitology. 2007;134:51–8. doi: 10.1017/S0031182006001272. [DOI] [PubMed] [Google Scholar]
- 55.Matin A, Siddiqui R, Jung SY, Kwang SK, Stins M, Khan NA. Balamuthia mandrillaris interactions with human brain microvascular endothelial cells in vitro. J Med Microbiol. 2007;56:1110–5. doi: 10.1099/jmm.0.47134-0. [DOI] [PubMed] [Google Scholar]
- 56.Mungroo MR, Khan NA, Siddiqui R. Balamuthia mandrillaris: Pathogenesis, diagnosis, and treatment. Expert Opin Orphan Drugs. 2020;8:111–9. [Google Scholar]
- 57.Matin A, Stins M, Kim KS, Khan NA. Balamuthia mandrillaris exhibits metalloprotease activities. FEMS Immunol Med Microbiol. 2006;47:83–91. doi: 10.1111/j.1574-695X.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- 58.Al Rokayan S, Razi Haider S. Colourimetric determination of phospholipase activities in Balamuthia mandrillaris. Am J Biochem Biotechnol. 2007;3:171–9. [Google Scholar]
- 59.Bravo FG, Alvarez PJ, Gotuzzo E. Balamuthia mandrillaris infection of the skin and central nervous system: An emerging disease of concern to many specialties in medicine. Curr Opin Infect Dis. 2011;24:112–7. doi: 10.1097/QCO.0b013e3283428d1e. [DOI] [PubMed] [Google Scholar]
- 60.Martínez AJ, Schuster FL, Visvesvara GS. Balamuthia mandrillaris: Its pathogenic potential. J Eukaryot Microbiol. 2001;48:6–10. doi: 10.1111/j.1550-7408.2001.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 61.Kodet R, Nohýnková E, Tichý M, Soukup J, Visvesvara GS. Amoebic encephalitis caused by Balamuthia mandrillaris in a Czech child: Description of the first case from Europe. Pathol Res Pract. 1998;194:423–9. doi: 10.1016/S0344-0338(98)80033-2. [DOI] [PubMed] [Google Scholar]
- 62.Tavares M, Correia da Costa JM, Carpenter SS, Santos LA, Afonso C, Aguiar A, et al. Diagnosis of first case of Balamuthia amoebic encephalitis in Portugal by immunofluorescence and PCR. J Clin Microbiol. 2006;44:2660–3. doi: 10.1128/JCM.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guarner J, Bartlett J, Shieh WJ, Paddock CD, Visvesvara GS, Zaki SR. Histopathologic spectrum and immunohistochemical diagnosis of amebic meningoencephalitis. Mod Pathol. 2007;20:1230–7. doi: 10.1038/modpathol.3800973. [DOI] [PubMed] [Google Scholar]
- 64.Schuster FL. Cultivation of pathogenic and opportunistic free-living amebas. Clin Microbiol Rev. 2002;15:342–54. doi: 10.1128/CMR.15.3.342-354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiderlen AF, Tata PS, Özel M, Laube U, Radam E, Schäfer H. Cytopathogenicity of Balamuthia mandrillaris, an opportunistic causative agent of granulomatous amoebic encephalitis. J Eukaryot Microbiol. 2006;53:456–63. doi: 10.1111/j.1550-7408.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 66.Lares-Jiménez LF, Gámez-Gutiérrez RA, Lares-Villa F. Novel culture medium for the axenic growth of Balamuthia mandrillaris. Diagn Microbiol Infect Dis. 2015;82:286–8. doi: 10.1016/j.diagmicrobio.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Schuster FL, Yagi S, Wilkins PP, Gavali S, Visvesvara GS, Glaser CA. Balamuthia mandrillaris, agent of amoebic encephalitis: Detection of serum antibodies and antigenic similarity of isolates by enzyme immunoassay. J Eukaryot Microbiol. 2008;55:313–20. doi: 10.1111/j.1550-7408.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- 68.Kiderlen AF, Radam E, Tata PS. Assessment of Balamuthia mandrillaris-specific serum antibody concentrations by flow cytometry. Parasitol Res. 2009;104:663–70. doi: 10.1007/s00436-008-1243-6. [DOI] [PubMed] [Google Scholar]
- 69.Huang ZH, Ferrante A, Carter RF. Serum antibodies to Balamuthia mandrillaris, a free-living amoeba recently demonstrated to cause granulomatous amoebic encephalitis. J Infect Dis. 1999;179:1305–8. doi: 10.1086/314731. [DOI] [PubMed] [Google Scholar]
- 70.Combs FJ, Jr, Erly WK, Valentino CM, Rance NE. Best cases from the AFIP: Balamuthia mandrillaris amebic meningoencephalitis. Radiographics. 2011;31:31–5. doi: 10.1148/rg.311105067. [DOI] [PubMed] [Google Scholar]
- 71.Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44:3589–95. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, et al. Real-time PCR in clinical microbiology: Applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiderlen AF, Radam E, Lewin A. Detection of Balamuthia mandrillaris DNA by real-time PCR targeting the RNase P gene. BMC Microbiol. 2008;8:1–6. doi: 10.1186/1471-2180-8-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yagi S, Booton GC, Visvesvara GS, Schuster FL. Detection of Balamuthia mitochondrial 16S rRNA gene DNA in clinical specimens by PCR. J Clin Microbiol. 2005;43:3192–7. doi: 10.1128/JCM.43.7.3192-3197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naccache SN, Peggs KS, Mattes FM, Phadke R, Garson JA, Grant P, et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clin Infect Dis. 2015;60:919–23. doi: 10.1093/cid/ciu912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson MR, Naccache SN, Samayoa E, Biagtan M, Bashir H, Yu G, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370:2408–17. doi: 10.1056/NEJMoa1401268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y, Hu X, Min L, Dong X, Guan Y. Balamuthia mandrillaris-related primary amoebic encephalitis in china diagnosed by next generation sequencing and a review of the literature. Lab Med. 2020;51:e20–6. doi: 10.1093/labmed/lmz079. [DOI] [PubMed] [Google Scholar]
- 78.Wu X, Yan G, Han S, Ye Y, Cheng X, Gong H, et al. Diagnosing Balamuthia mandrillaris encephalitis via next-generation sequencing in a 13-year-old girl. Emerg Microbes Infect. 2020;9:1379–87. doi: 10.1080/22221751.2020.1775130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalyatanda G, Rand K, Lindner MS, Hong DK, Sait Albayram M, Gregory J, et al. Rapid, noninvasive diagnosis of Balamuthia mandrillaris encephalitis by a plasma-based next-generation sequencing test. Open Forum Infect Dis. 2020;7:ofaa189. doi: 10.1093/ofid/ofaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy SL, Atkins JT, Gennuso R, Kofos D, Sriram RR, Dorlo TP, et al. Assessment of blood-brain barrier penetration of miltefosine used to treat a fatal case of granulomatous amoebic encephalitis possibly caused by an unusual Balamuthia mandrillaris strain. Parasitol Res. 2015;114:4431–9. doi: 10.1007/s00436-015-4684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: Presentation of 2 cases. Clin Infect Dis. 2003;37:1304–12. doi: 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- 82.Lehmer LM, Ulibarri GE, Ragsdale BD, Kunkle J. Cutaneous Balamuthia mandrillaris infection as a precursor to Balamuthia amoebic encephalitis (BAE) in a healthy 84-year-old Californian. Dermatol Online J. 2017;23:1–10. [PubMed] [Google Scholar]
- 83.Martínez DY, Seas C, Bravo F, Legua P, Ramos C, Cabello AM, et al. Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis. 2010;51:7–11. doi: 10.1086/653609. [DOI] [PubMed] [Google Scholar]
- 84.Kalsoom H, Baig AM, Khan NA, Siddiqui R. Laboratory testing of clinically approved drugs against Balamuthia mandrillaris. World J Microbiol Biotechnol. 2014;30:2337–42. doi: 10.1007/s11274-014-1658-4. [DOI] [PubMed] [Google Scholar]
- 85.Laurie MT, White CV, Retallack H, Wu W, Moser MS, Sakanari JA, et al. Functional assessment of 2,177 U.S. and international drugs identifies the quinoline nitroxoline as a potent amoebicidal agent against the pathogen Balamuthia mandrillaris. mBio. 2018;9:1–6. doi: 10.1128/mBio.02051-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kangussu-Marcolino MM, Ehrenkaufer GM, Chen E, Debnath A, Singh U. Identification of plicamycin, TG02, panobinostat, lestaurtinib, and GDC-0084 as promising compounds for the treatment of central nervous system infections caused by the free-living amoebae Naegleria, Acanthamoeba and Balamuthia. Int J Parasitol Drugs Drug Resist. 2019;11:80–94. doi: 10.1016/j.ijpddr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mungroo MR, Shahbaz MS, Anwar A, Saad SM, Khan KM, Khan NA, et al. Aryl quinazolinone derivatives as novel therapeutic agents against brain-eating amoebae. ACS Chem Neurosci. 2020;11:2438–49. doi: 10.1021/acschemneuro.9b00596. [DOI] [PubMed] [Google Scholar]
- 88.Intalapaporn P, Suankratay C, Shuangshoti S, Phantumchinda K, Keelawat S, Wilde H. Balamuthia mandrillaris meningoencephalitis: The first case in Southeast Asia. Am J Trop Med Hyg. 2004;70:666–9. [PubMed] [Google Scholar]
- 89.Jung S, Schelper RL, Visvesvara GS, Chang HT. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: An unusual clinical course and a favorable outcome. Arch Pathol Lab Med. 2004;128:466–8. doi: 10.5858/2004-128-466-BMMIAI. [DOI] [PubMed] [Google Scholar]
- 90.Pietrucha-Dilanchian P, Chan JC, Castellano-Sanchez A, Hirzel A, Laowansiri P, Tuda C, et al. Balamuthia mandrillaris and Acanthamoeba amoebic encephalitis with neurotoxoplasmosis coinfection in a patient with advanced HIV infection. J Clin Microbiol. 2012;50:1128–31. doi: 10.1128/JCM.06252-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16S rRNA gene as a target. J Clin Microbiol. 2003;41:453–5. doi: 10.1128/JCM.41.1.453-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bakardjiev A, Azimi PH, Ashouri N, Ascher DP, Janner D, Schuster FL, et al. Amebic encephalitis caused by Balamuthia mandrillaris: Report of four cases. Pediatr Infect Dis J. 2003;22:447–52. doi: 10.1097/01.inf.0000066540.18671.f8. [DOI] [PubMed] [Google Scholar]
- 93.Yamanouchi K, Arima H, Sakamoto Y, Kanto K, Kasai K, Ito K, et al. First report of the isolation of Balamuthia mandrillaris in the northern region of Japan. Parasitol Res. 2018;117:2895–900. doi: 10.1007/s00436-018-5980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lares-Jiménez LF, Booton GC, Lares-Villa F, Velázquez-Contreras CA, Fuerst PA. Genetic analysis among environmental strains of Balamuthia mandrillaris recovered from an artificial lagoon and from soil in Sonora, Mexico. Exp Parasitol. 2014;145 Suppl:S57–61. doi: 10.1016/j.exppara.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 95.Khurana S, Hallur V, Goyal MK, Sehgal R, Radotra BD. Emergence of Balamuthia mandrillaris meningoencephalitis in India. Indian J Med Microbiol. 2015;33:298–318. doi: 10.4103/0255-0857.154887. [DOI] [PubMed] [Google Scholar]


