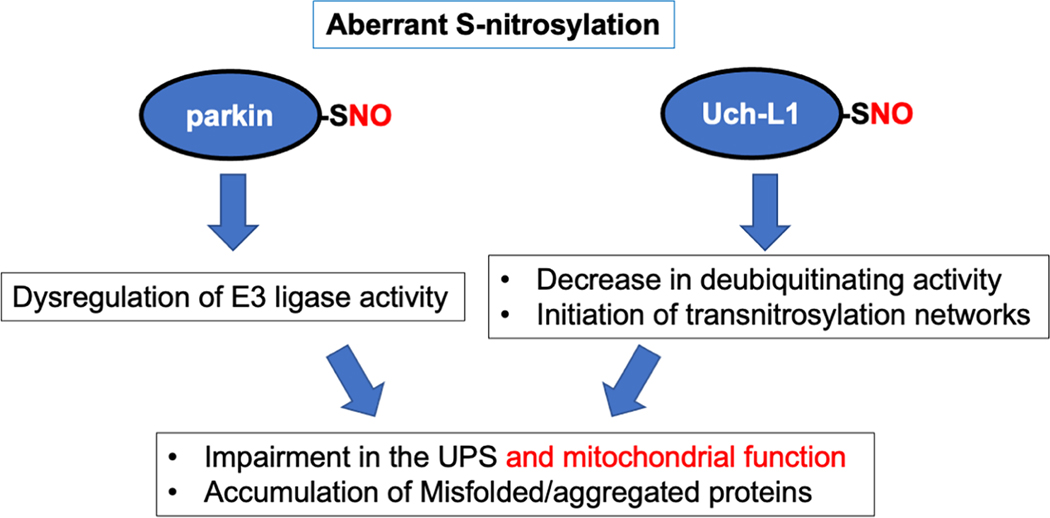
Fig. 2.
S-Nitrosylation of PDI impairs its chaperone and disulfide isomerase activity that modulates protein folding. When an excessive amount of NO is present under pathological conditions, PDI is S-nitrosylated at its active site cysteines, thus inhibiting its protein folding and chaperone activity, resulting in accumulation of misfolded proteins with increased cell damage and death.