FIG 1.
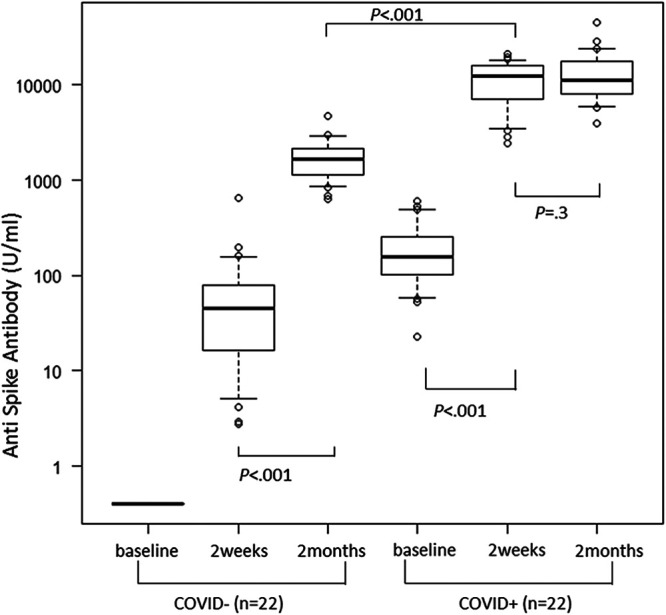
Antibody responses in age- and sex-matched pairs with or without a history of COVID-19. All participants except four (one, anaphylaxis following the first dose; three, quit their job) received two doses of BNT162b2 vaccine; the second dose was administered 18 to 25 days after the first dose was administered. Plasma was drawn before vaccination (day −7 to 0), at 2 weeks (day 15 to 21) after vaccination, and at 2 months (day 57 to 64) after vaccination. Antibody titers against the receptor binding domain of the SARS-CoV-2 S1 subunit of the spike protein were determined using Elecsys anti-SARS-CoV-2 S (Roche Diagnostics International Ltd., Rotkreuz, Switzerland). In cases wherein the detection limit (250 U/mL) was exceeded, plasma samples were diluted 50 to 200 times, as appropriate. To minimize confounding, 22 age- and sex-matched individuals were randomly identified (COVID−) among the uninfected individuals (n = 343), after excluding 4 seropositive individuals. Data of previously infected individuals (COVID+; n = 22) and those of COVID− participants were compared. Both groups showed elevation of antibody titers after the first dose. Following the second dose, titers in COVID− individuals were boosted, but titers for COVID+ individuals did not increase at 2 months. The antibody titers after 2 months in COVID− individuals were higher than the prevaccination antibody titer in COVID+ individuals but lower than the antibody titer at 2 weeks in COVID+ individuals.
