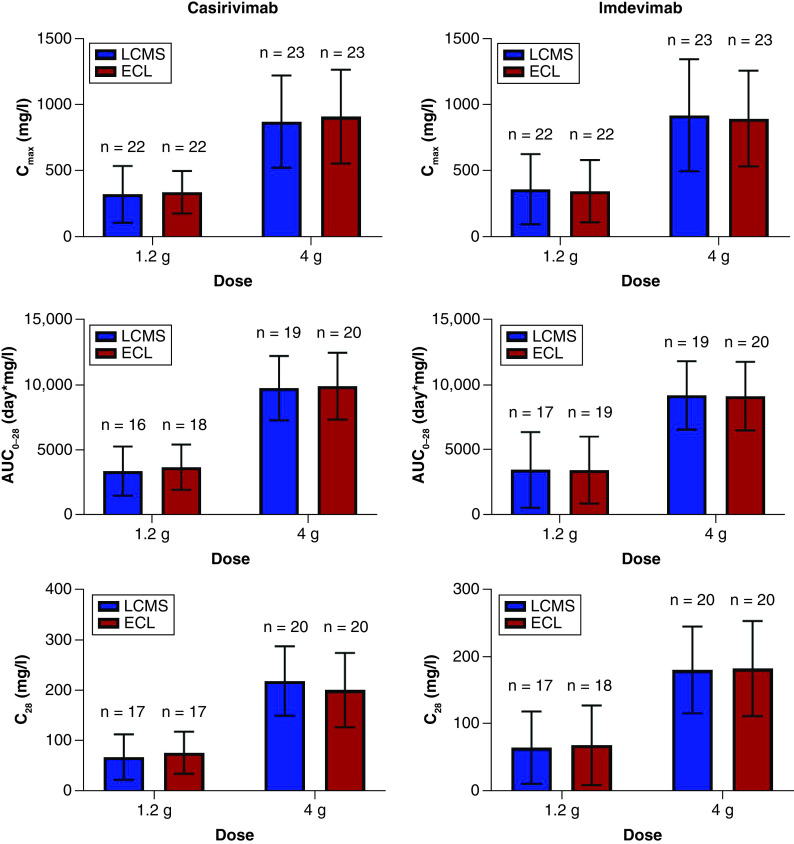
Figure 5. Pharmacokinetic parameters per platform for each casirivimab (left) and imdevimab (right) in non-hospitalized patients with SARS-CoV-2 diagnosis (Study 2067).
For the 1.2 g and 4 g doses per mAb, the following parameters are presented as mean ± standard deviation (SD) with number of subjects identified for each calculation (n): (top) maximum drug concentration in blood (Cmax), (middle) area under concentration by time curve up to 28 days post-dose (AUC0–28), and (bottom) drug concentration in serum at 28 days after dose (C28).