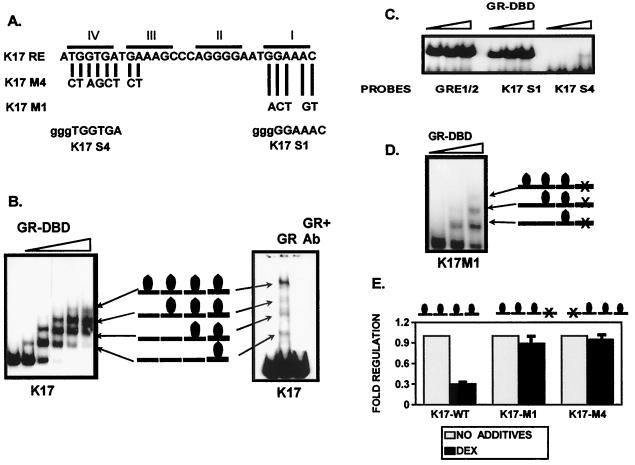
FIG. 7.
Four monomers of GR bind to the keratin GREs and mediate their suppression. (A) Summary of the sequence analysis. Introduced mutations used in cotransfection experiments are shown as K17M1 and K17M4. K17S4 and K17S1 probes for the gel shift experiment are shown below with 5′ GGG overhang designed for labeling. (B) Gel shift experiment with recombinant GR-DBD (left), full-size GR (right), and a K17RE probe is shown. GR-DBD binds initially as a monomer. As the concentration of the GR-DBD increases, two monomers, then three, and finally four are bound to the GREs. A gel shift experiment with recombinant human full-size GR and a K17RE probe shows a similar binding pattern. A monoclonal GR-specific antibody raised against the DBD region blocks the binding. (C) The primary and quaternary binding sites in the K17GRE have different affinities of binding to GR. GR binds with similar affinities to the consensus GRE half-site (GRE1/2) and the primary binding site in K17GRE (K17S1), whereas the quaternary site (K17S4) binds with a significantly lower affinity in gel shift experiments. (D) Binding of the GR to four binding sites in keratin GRE is not cooperative. Mutation in the primary binding site K17M1 did not affect the binding of the GR to the remaining three binding sites in keratin K17GRE in the gel shift experiments. (E) The binding of all four monomers is required for the suppression of keratin gene transcription. Mutations introduced into the primary (K17M1) or the quaternary (K17M4) binding sites abolished regulation by DEX in cotransfection experiments.