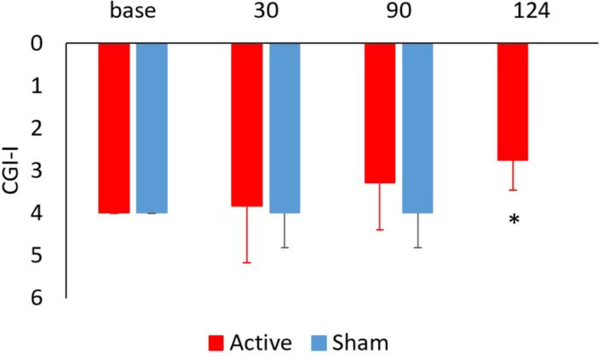
Figure 7.
Effects of tcVNS and sham on clinical improvement as measured with the Clinical Global Impressions scale-improvements (CGI-I) in active tcVNS (red) and sham (blue) stimulation groups at baseline and 30 and 90 days after start of double-blind active (N=9) versus sham (N=11) treatment in patients with PTSD. The final measurement at 124 days (34 days after start of open label treatment) showed a significant improvement compared to after three months of sham stimulation (2.75 (0.71) versus 4.00 (0.82 SD) (*p=0.003). This score is intermediated between much improved (2) and minimally improved (3) compared to no improvement (4).