Reaction optimization studies.
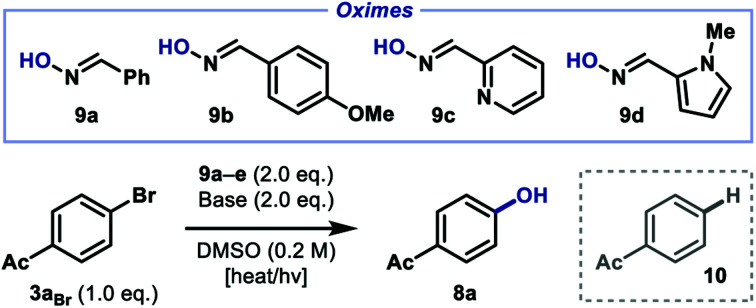
| |||||
|---|---|---|---|---|---|
| Entrya | Oxime | Temp./hv | Base | Time | Yieldb 8a/% |
| 1 | 9a | 30 °C | KOt-Bu | 16 h | 52 |
| 2 | 9b | 30 °C | KOt-Bu | 16 h | 45 |
| 3 | 9c | 30 °C | KOt-Bu | 16 h | 38 |
| 4 | 9d | 30 °C | KOt-Bu | 16 h | 75 |
| 5 | 9d | 30 °C | KOH | 16 h | 38 |
| 6 | 9d | 30 °C | Cs2CO3 | 16 h | 25 |
| 7c | 9d | 450 nm | KOt-Bu | 1 h | 65 |
| 8d | 9d | 30 °C | KOt-Bu | 1 h | 38 |
| 9 | 9d | 30 °C | KOt-Bu | 1 h | 44 |
| 10e | 9d | 30 °C | KOt-Bu | 16 h | 10 |
| 11f | 9d | 30 °C | KOt-Bu | 16 h | <5 |
| 12g | 9d | 30 °C | KOt-Bu | 16 h | 49 |
Reactions performed with 0.1 mmol of aryl bromide 3aBr and 0.2 mmol oxime 9a–d with the stated base (0.2 mmol) in DMSO (0.5 mL) under nitrogen.
Determined by 1H NMR spectroscopy against an internal standard (dibromomethane).
Under irradiation with 18 W blue LEDs (λmax = 450 nm) and fan cooling.
Reaction performed in the dark.
Reaction performed in the presence of galvinoxyl (1 equiv.).
Reaction performed in the presence of DPPH (1 equiv.).
Reaction performed in the presence of TEMPO (2 equiv.).
