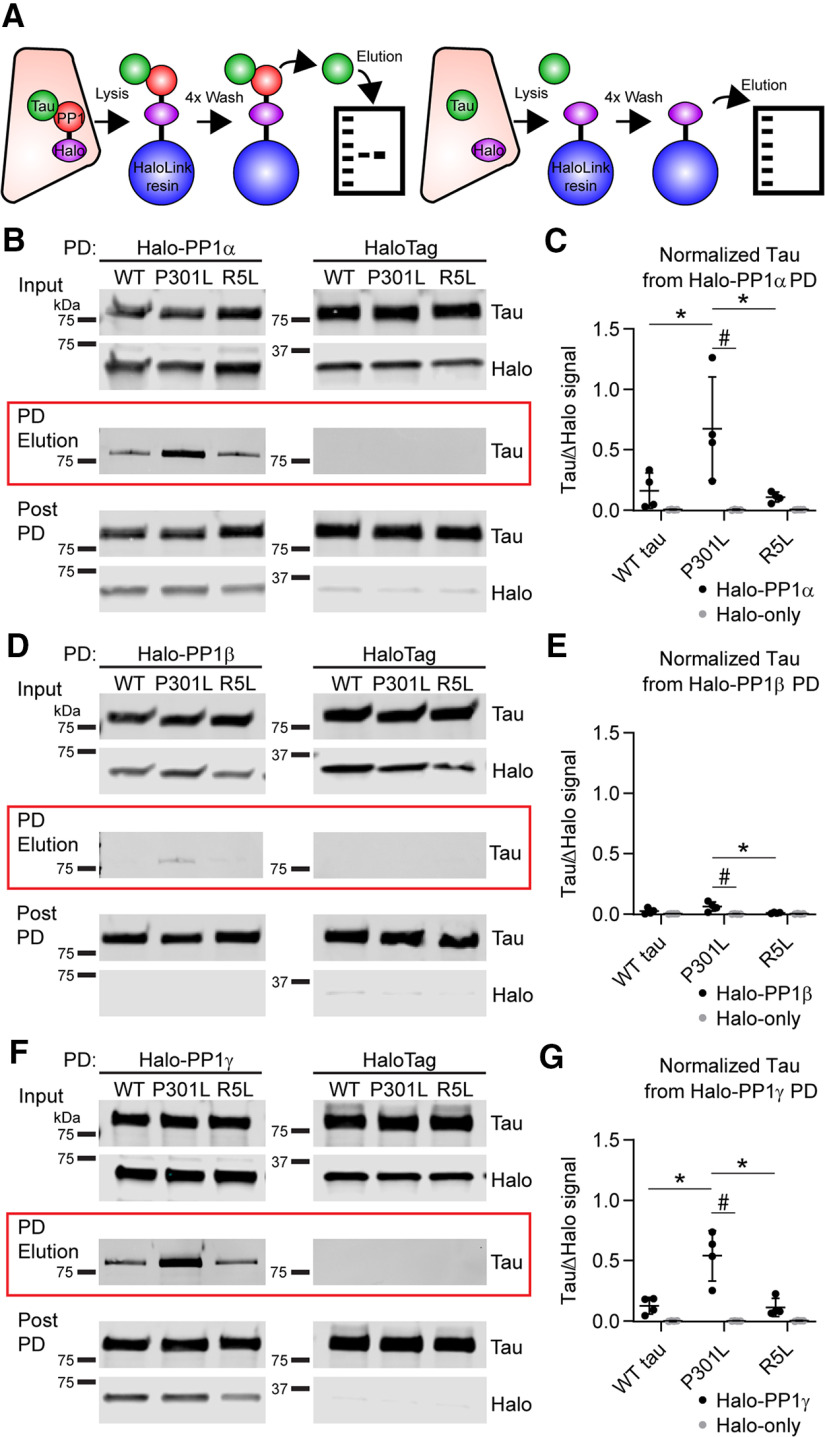
Figure 2.
P301L mutation in tau enhances the interaction with PP1α and γ isoforms in pull-down (PD) assays. A, Schematic showing assay design. Individual tau constructs were coexpressed with HaloTag-PP1 (left) or HaloTag-only controls (right) in HEK293T cells. Lysates prepared from these cells were incubated with HaloLink resin, which covalently binds to HaloTag. We quantified the amount of tau signal (pan-tau R1 antibody, green) eluted from each pull down by immunoblot (PD elution) and normalized to the reduction in Halo signal (anti-Halo antibody, red) from prebinding to postbinding lysate samples. B, WT, P301L tau, and R5L tau proteins were detected in the elution samples after a pull down with Halo-PP1α (left, red box). Tau was not detected in the HaloTag-only control (right, red box). Lysate samples obtained before (Input) and after pull down with the HaloLink resin (Post PD) were probed to confirm similar protein expression and HaloTag binding to resin. C, Quantitation of tau in the elution samples indicate that P301L tau (0.673 ± 0.429) significantly increases the interaction with PP1α when compared with WT (0.160 ± 0.147) and R5L tau (0.107 ± 0.041) and the control with HaloTag-only (WT tau, 0.004 ± 0.002; P301L, 0.003 ± 0.003; R5L, 0.003 ± 0.001). D, A relatively low level of WT and P301L tau was eluted from the Halo-PP1β pull down but not the HaloTag-only control. The pre- and post-pull-down lysate samples from the Halo-PP1β and Halo-only lysates confirm similar expression and pull-down efficiency. E, Quantitation of tau in the pull-down elution samples indicate that P301L tau (0.061 ± 0.038) significantly increases the interaction with PP1β when compared with the P301L + HaloTag control (−0.001 ± 0.001). Notably, the amount of tau in the elution with Halo-PP1β pull down is much lower for all forms (WT tau, 0.023 ± 0.024; R5L, 0.009 ± 0.005) and not significantly higher than the HaloTag-only controls (WT tau, 0.001 ± 0.001; R5L, −0.001 ± 0.001). F, WT and P301L tau proteins were detected in the elution samples after a pull down with Halo-PP1γ. The pre- and post-pull-down lysate samples from the Halo-PP1γ and Halo-only lysates confirm similar expression and pull-down efficiency. G, Quantitation of tau in the elution samples indicate that P301L tau (0.540 ± 0.209) significantly increases the interaction with PP1γ when compared with WT tau (0.125 ± 0.069), R5L tau (0.111 ± 0.075), and the P301L + HaloTag control (0.000 ± 0.001). Tau was also absent in HaloTag pull-down controls (WT tau, −0.001 ± 0.002; R5L, 0.001 ± 0.002). All data in the legend are reported as ratios of the signal intensities in arbitrary units. Statistical comparisons were performed using a repeated measures two-way ANOVA with Tukey's multiple comparison test, and each data point represents an independent experimental replicate (n = 4 independent replicates; data are mean ± SD; * p ≤ 0.05 for comparisons among the tau group; #p ≤ 0.05 for the comparison between Halo-PP1 and Halo-only pull downs).