Supplemental Digital Content is available in the text.
Keywords: antimicrobial stewardship, cost, critical illness, outcomes, procalcitonin, sepsis
OBJECTIVES:
To examine the impact before and after adoption of a procalcitonin-based protocol to guide sepsis management has on antibiotic use, care costs, and outcomes of critically ill patients.
DESIGN:
Before-after study.
SETTING:
ICU of an academic tertiary care center.
PATIENTS:
Adults over 18 years old admitted to the ICU from January 1, 2017, to January 31, 2020.
INTERVENTIONS:
In this before-after study, we compared the use of medications, outcomes, and overall cost before and after the introduction of a procalcitonin-based protocol for evaluation and treatment of sepsis.
MEASUREMENTS AND MAIN RESULTS:
The final study cohort consisted of 1,793 patients admitted to the ICU, 776 patients pre-procalcitonin and 1,017 patients in the post-procalcitonin period. Patients were not different in the pre-procalcitonin adoption period compared with post-procalcitonin adoption with regard to gender, age (62.0 vs 62.6), race, or comorbidities. Patients admitted during the post-procalcitonin adoption period were less likely to receive the examined broad-spectrum antibiotics (odds ratio, –0.58; CI, –0.99 to –0.17; p < 0.01) than patients during the pre-procalcitonin adoption period. The odds of inhospital death did not differ after procalcitonin adoption when compared with before (0.87; CI, 0.70–1.09; p = 0.234). Total charges for each admission were significantly less in the post-procalcitonin adoption period $3,834.99 compared with pre-procalcitonin adoption $4,429.47 (p < 0.05). Patients post-procalcitonin adoption incurred $1,127.18 per patient less in total charges (–1,127.18; CI, –2,014.74 to –239.62; p = 0.013) after controlling for relevant factors.
CONCLUSIONS:
In critically ill patients in a large U.S. tertiary care hospital, the adoption of a procalcitonin-based protocol for evaluation and treatment of sepsis may be associated with decreased antibiotic use and significant cost savings, with no change in mortality.
Sepsis is the single most expensive condition treated in U.S. hospitals (1, 2). Although early initiation of antibiotics improves outcomes in sepsis (3–6), decisions to continue or stop antibiotic administration have traditionally relied upon clinician experience, patient condition, and culture data. Unfortunately, clinical tests for sepsis such as systemic inflammatory response syndrome (SIRS) criteria, quick Sepsis-related Organ Failure Assessment score, and lactate do not effectively identify the presence of bacterial infection. In addition, outcomes of antibiotic treatment for culture-negative sepsis do not differ from culture-positive sepsis, suggesting that blood culture results also poorly predict the response to antibiotics (7, 8).
Procalcitonin is an amino acid precursor of calcitonin that is secreted by thyroid C cells and can also be produced in response to bacterial lipopolysaccharide and inflammatory mediators. With the onset of bacterial infection, procalcitonin is detectable within 2–6 hours with peak elevation usually at 6–24 hours. It has a half-life of approximately 24 hours, which is reflected in a rapid decline with control of infection. In clinical studies, procalcitonin decreases antimicrobial usage without negative impact on clinical outcomes (9–16).
The Healthcare Cost and Utilization Project and the Agency for Healthcare Research and Quality have highlighted the considerable morbidity, mortality, and cost of sepsis care in the United States (1, 2). Any improvement to sepsis management can significantly improve patient care and costs. The objective of this study is to examine the impact of a clinical intervention that consists of adoption of in-house procalcitonin availability and a protocol for physician ordering in one U.S. tertiary health system on cost, antibiotic use, and clinical outcomes. We postulate that physicians in the ICU will use procalcitonin tests as needed to guide appropriate antibiotic initiation and discontinuation of treatment, which will lead to lower use and costs of antibiotic treatment, cultures, and vasoactive medications without adversely affecting clinical outcomes. Therefore, in our analysis, appropriate use of procalcitonin is a mediator in the relationship between the adoption of the clinical intervention and cost and clinical outcomes. To test our hypothesis, we reviewed antibiotic use, care costs, and outcomes of critically ill patients before and after introduction of a protocol for procalcitonin testing to guide sepsis management.
METHODS
Sample Characteristics
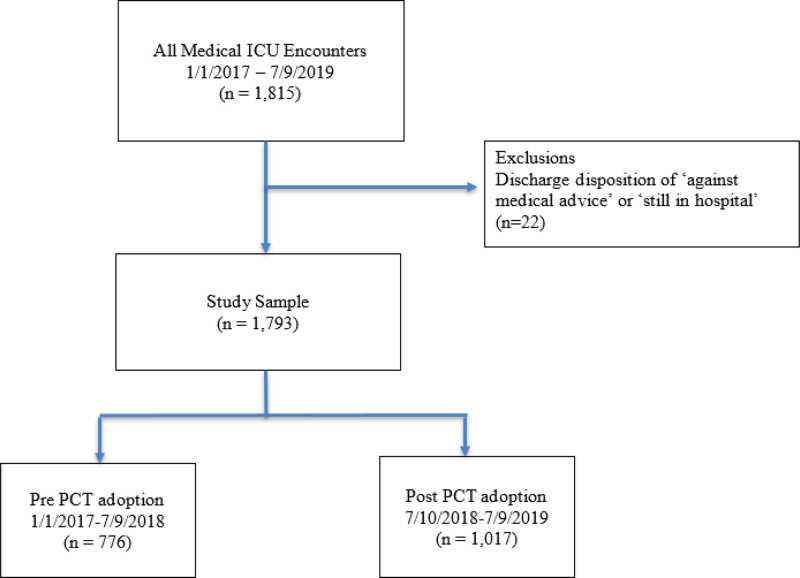
After approval from the Loyola University Chicago Institutional Review Board (Number 211354), we performed a single-center retrospective study of all medical ICU adult encounters at a 547-bed tertiary academic medical center between January 1, 2017, and January 31, 2020. Eligible encounters included all patients 18 years old or older. Patients were excluded if they had a discharge disposition of “against medical advice” and “still in hospital” (n = 22). The final study sample included 776 patients in the pre-procalcitonin adoption period and 1,017 in the post-procalcitonin adoption period, resulting in a total of 1,793 encounters (Fig. 1).
Figure 1.
Flow chart diagram of medical ICU encounters pre- and post-hospital-wide adoption of the procalcitonin (PCT) test. All encounters for admission into the medical ICU were included. Exclusion criteria included a discharge disposition of “against medical advice” or “still in hospital.”
Hospital Wide Adoption of the Procalcitonin
We defined the pre-procalcitonin adoption period from January 1, 2017, to July 9, 2018, and the post-procalcitonin adoption period from July 10, 2018, to January 31, 2020. We chose these time periods because procalcitonin became an available laboratory in-house assay on July 10, 2018, with results available within 2 hours. In June 2018, we implemented a procalcitonin protocol to provide guidance in the management of lower respiratory tract infections (LRTIs) and suspected bacterial sepsis. For LRTI, we provided guidance based on the following values: less than or equal to 0.25 ng/mL = bacterial etiology highly unlikely, antibiotics strongly discouraged; 0.25–0.5 ng/mL = antibiotic therapy dependent upon clinical suspicion for infection, recommend retest 12 hours after initial draw; greater than 0.5 ng/mL = bacterial etiology highly likely, antibiotics strongly encouraged. For suspected bacterial sepsis: less than 0.5 ng/mL = sepsis not likely, local bacterial infection possible, suggest retest 12 hours after initial draw; 0.5–2.0 ng/mL = sepsis possible, but other conditions may also elevate procalcitonin, suggest retest 12 hours after initial draw; greater than 2.0 ng/mL = sepsis is likely (Supplemental Figure, http://links.lww.com/CCX/A842). Procalcitonin testing was incorporated into the emergency department sepsis bundle and house staff were educated during orientation for the 2018 new academic year. Prior to this time, procalcitonin measurements were performed at an outside facility, cost over 10-fold more than the standardized in-house assay, and required a 10-day wait for results.
Patient Data
Demographic factors queried from the electronic health record (EHR) included patient’s age, gender, race (Black, White and Asian, or other), ethnicity (Hispanic vs non-Hispanic), and payer status (Medicaid or other government, Medicare, private insurance, and self-pay or uninsured). Other race category included American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander. The number of ICU days and total number of hospital days during the hospitalization were also queried. Comorbidities were ascertained using the International Classification of Diseases, 10th Revision, Clinical Medication diagnosis codes for the encounter using the Elixhauser index (17).
Clinical Data: Cultures, Medications, Charges, and Procalcitonin Use
Information on cultures, laboratories, and medications dispensed was obtained from the EHR. The number of procalcitonin ordered was calculated per encounter. Antibiotic and vasoactive medication use was defined as the total number of days a patient received the medication. We also defined antibiotic use as the total number of days of antibiotic used. Antibiotics examined included cefepime, vancomycin, metronidazole, piperacillin-tazobactam, and meropenem as the standard antibiotics included in empiric regimens while awaiting laboratory results. Vasoactive medications included norepinephrine, vasopressin, epinephrine, dopamine, dobutamine, and phenylephrine. Culture sources included blood, sputum, and urine. Total charges included charges for procalcitonin, cultures, antibiotics, and vasoactive medications. Since our study timeline spans over multiple years and the nominal amount of money tend to increase over time, the Hospital Care component of the Personal Healthcare Price Index published by Centers for Medicare and Medicaid Services was used to adjust 2018–2019 hospital charges to 2020 U.S. dollars (18, 19). Since the inflation rate between 2018 and 2019 is most recently available, a similar rate of inflation was assumed for 2019–2020.
Adverse Outcomes, Readmissions, and Discharge Disposition
Each patient’s first and last creatinine value was collected for each encounter. The total number of days a patient required renal replacement therapy or mechanical ventilation was also calculated. Same-hospital 30-day readmissions to the ICU or to the hospital were calculated. Discharge dispositions included discharge to home, nursing home or skilled nursing facility, or death.
Statistical Analysis
Bivariate descriptive statistics were conducted to compare sociodemographic and clinical characteristics of medical ICU adult encounters pre- and post-procalcitonin adoption. Categorical variables were compared using the chi-square test and continuous variables were compared using the t test for normally distributed variables and the nonparametric K-sample test on the equality of medians for non-normally distributed data. Similar statistics were conducted to compare the utilization, charges, and outcome variables.
Since days of antibiotics used and length of stay (LOS) are non-normally distributed, the multivariate quantile median regression model was performed to examine the effect of procalcitonin adoption on antibiotic usage. The quantile median regression model expresses the median of the conditional distribution of the outcome as a linear function of the independent variables (1). The coefficient value represents the median change in the outcome given a unit or group change in the predictor.
Inhospital death and 30-day readmissions are binary variables; therefore, the multivariate logistic regression analysis was conducted to examine the odds of inhospital death and 30-day readmission (among patients with no inhospital death) post- versus pre-procalcitonin adoption period. Results are displayed in odds ratios with CIs. As for the charges model, a generalized linear model with gamma family and log function was used to estimate changes in cost among encounters post- versus pre-procalcitonin adoption period.
All regression analysis adjusted for patient demographic and clinical characteristics listed in the Supplemental Table (http://links.lww.com/CCX/A843). Since 16.18% of our patients had more than one encounter during the study period, all analysis was conducted by clustering at the patient level. Missing race and ethnicity data were categorized separately for regression purposes. Encounters with missing charges for antibiotics or vasoactive medications were not included in analyses of cost. For the statistical tests used in this study, all reported p values were two-sided, and statistical significance was defined as alpha = 0.05. Analyses were conducted using Stata MP Software, Version 15.1 (StataCorp LP, College Station, TX).
RESULTS
Of the 1,793 patients included in the final dataset, there were 776 patients in the pre-procalcitonin adoption period and 1,017 in the post-procalcitonin period (Fig. 1). There were 53.0% male patients in the pre-procalcitonin adoption period, similar to 52.7% in the post-procalcitonin adoption period (Table 1). Additionally, there was no significant difference in age (62.0 vs 62.6), race, or comorbidities. Patients were more likely to have Medicaid insurance pre-procalcitonin adoption (152, 19.6%) compared with post-procalcitonin adoption (155, 15.3%); however, patients were otherwise similar with days in the ICU, hospital LOS, and comorbidities (Table 1).
TABLE 1.
Sociodemographic and Clinical Characteristics of Medical ICU Adult Encounters Pre- and Post-Procalcitonin Test Adoption Period (n = 1,793)
| Characteristic | Pre-Procalcitonin Adoption (n = 776) | Post-Procalcitonin Adoption (n = 1,017) | p |
|---|---|---|---|
| Age, mean (± sd) | 62.0 ± 16.1 | 62.6 ± 16.2 | 0.427 |
| Gender, male n (%) | 411 (53.0) | 536 (52.7) | 0.913 |
| Race, n (%) | 0.910 | ||
| White | 438 (56.5) | 584 (57.6) | |
| Black | 229 (29.6) | 285 (28.1) | |
| Asian or other | 105 (13.6) | 140 (13.8) | |
| Ethnicity, n (%) | 0.040 | ||
| Hispanic | 87 (11.2) | 145 (14.3) | |
| Non-Hispanic | 681 (87.8) | 852 (83.8) | |
| Insurance, n (%) | 0.065 | ||
| Medicaid or other government | 152 (19.6) | 155 (15.3) | |
| Medicare | 416 (53.6) | 598 (58.9) | |
| Private | 198 (25.5) | 252 (24.8) | |
| Self-pay or uninsured | 10 (1.3) | 11 (1.1) | |
| Days in ICU, median (IQR) | 5 (3–9) | 5 (3–8) | 0.677 |
| Hospital length of stay, median (IQR) | 10 (6–20) | 11 (6–21) | 0.163 |
| Elixhauser comorbidity index, n (%) | 0.520 | ||
| 0 comorbidities | 5 (0.6) | 7 (0.7) | |
| 1–4 comorbidities | 193 (24.9) | 264 (26.0) | |
| 5–8 comorbidities | 436 (56.2) | 587 (57.7) | |
| 9–31 comorbidities | 142 (18.3) | 159 (15.6) |
IQR = interquartile range.
Column percent do not total 100% due to missing values (< 2%) in the race and ethnicity variables.
In the pre-procalcitonin adoption period, procalcitonin was rarely used, 97.7% patients having not had any procalcitonin laboratories ordered, compared with 38.3% in the post-procalcitonin adoption period. In the post-procalcitonin adoption period, 61.8% patients had at least one procalcitonin test ordered and therefore more charges were associated with procalcitonin tests during the post-procalcitonin adoption period. However, when overall charges including procalcitonin tests, culture tests, antibiotic, and vasoactive medication use were compared total charges were significantly less in the post-procalcitonin adoption period, $3,834.99 compared with $4,429.47 in the pre-procalcitonin adoption period (Table 2). Patients in the pre-procalcitonin and post-procalcitonin adoption periods had similar clinical outcomes with respect to days requiring renal replacement therapy, mechanical ventilation, and 30-day readmissions (Table 3).
TABLE 2.
Procalcitonin Test, Culture, Antibiotics, and Vasoactive Medications Use and Charges of Medical ICU Adult Encounters Pre- and Post-Procalcitonin Adoption Period (n = 1,793)
| Variables | Pre-Procalcitonin Adoption (n = 776) | Post-Procalcitonin Adoption (n = 1,017) | p |
|---|---|---|---|
| Utilization | |||
| Procalcitonin (%) | < 0.001 | ||
| None | 758 (97.68) | 389 (38.25) | |
| One | 13 (1.68) | 407 (40.02) | |
| Two | 3 (0.39) | 127 (12.49) | |
| Three or more | 2 (0.26) | 94 (9.24) | |
| Culture orders | |||
| Blood, median (IQR) | 2 (1–4) | 2 (0–3) | < 0.001 |
| Sputum, median (minimum–maximum) | 0 (0–8) | 0 (0–7) | 0.066 |
| Urine, median (IQR) | 2 (1–3) | 2 (1–3) | 0.242 |
| Antibiotics | |||
| Days, median (IQR) | 8 (4–14) | 7 (4–13) | 0.310 |
| Cefepime days, median (IQR) | 1 (0–4) | 1 (0–4) | < 0.05 |
| Vancomycin days, median (IQR) | 4 (2–8) | 3 (1–7) | 0.364 |
| Metronidazole days, median (IQR) | 1 (0–5) | 0 (0–4) | < 0.001 |
| Piperacillin/tazobactam days, median (IQR) | 0 (0–5) | 1 (0–6) | 0.017 |
| Meropenem days, median (IQR) | 0 (0–3) | 0 (0–0) | < 0.001 |
| Vasoactive medications | |||
| Norepinephrine days, median (minimum–maximum) | 2 (0–30) | 1 (0–38) | 0.201 |
| Vasopressin days, median (minimum–maximum) | 0 (0–28) | 0 (0–38) | 0.206 |
| Epinephrine days, median (minimum–maximum) | 2 (0–35) | 1 (0–38) | 0.243 |
| Dopamine days, median (minimum–maximum) | 0 (0–18) | 0 (0–24) | 0.750 |
| Dobutamine days, median (minimum–maximum) | 0 (0–17) | 0 (0–50) | 0.194 |
| Phenylephrine days, median (minimum–maximum) | 0 (0–9) | 0 (0–31) | 0.026 |
| Charges in 2020 U.S. $ | |||
| Procalcitonin, median (minimum–maximum) | 0 (0–856.11) | 36.10 (0–649.93) | < 0.001 |
| Total culture ordersa, median (IQR) | 954.71 (618.00–1,654.70) | 677.27 (320.27–1,121.98) | < 0.001 |
| Total antibioticsb, median (IQR) | 2,815.35 (1,145.31–5,533.78) | 2,278.72 (909.06–4,331.91) | < 0.05 |
| Total vasoactive medicationsc, median (IQR) | 219.01 (0–911.66) | 230.09 (0–1,494.52) | 0.961 |
| Total chargesd, median (IQR) | 4,429.47 (2,359.94–8,659.47) | 3,834.99 (1,875.07–7,708.47) | < 0.05 |
IQR = interquartile range.
aTotal culture orders charges include charges for blood, sputum, and urine cultures.
bTotal antibiotics charges include charges for cefepime, vancomycin, metronidazole, piperacillin/tazobactam, and meropenem days.
cTotal vasoactive medications charges include charges for norepinephrine, vasopressin, epinephrine, dopamine, dobutamine, and phenylephrine days.
dTotal charges include charges for procalcitonin, cultures, antibiotics, and vasoactive medications use.
Minimum and maximum are reported instead of IQR for variables with low utilization.
TABLE 3.
Adverse Outcomes, Readmissions, and Discharge Disposition of Medical ICU Adult Encounters Pre- and Post-Procalcitonin Test Adoption Period (n = 1,793)
| Outcomes | Pre-Procalcitonin Adoption (n = 776) | Post-Procalcitonin Adoption (n = 1,017) | p |
|---|---|---|---|
| Average creatinine level, mean (se) | 2.02 (0.06) | 1.96 (0.05) | 0.438 |
| First creatinine level, mean (se) | 2.29 (0.08) | 2.11 (0.06) | 0.080 |
| Last creatinine level, mean (se) | 1.90 (0.06) | 1.84 (0.05) | 0.456 |
| Days on dialysis or renal replacement therapy, median (minimum–maximum) | 0 (0–39) | 0 (0–115) | 0.213 |
| Days on mechanical ventilation, median (minimum–maximum) | 1 (0–99) | 0 (0–137) | 0.671 |
| 30-d same facility readmissions (%) | 0.059 | ||
| None | 387 (71.27) | 568 (75.94) | |
| One or more | 156 (28.73) | 180 (24.06) | |
| Discharge disposition (%) | 0.246 | ||
| Home | 275 (35.44) | 381 (37.46) | |
| Facility | 268 (34.54) | 367 (36.09) | |
| Expired | 233 (30.03) | 269 (26.45) |
Minimum and maximum are reported instead of interquartile range for variables with low utilization.
Patients managed during the post-procalcitonin adoption period were less likely to receive the studied antibiotics (coefficient, –0.58; CI, –0.99 to –0.17; p < 0.01) than patients during the pre-procalcitonin adoption period (Supplemental Table, http://links.lww.com/CCX/A843). Other factors significantly associated with lower median antibiotic days included being female (–0.64; CI, –1.05 to –0.23; p < 0.01) and longer days on renal replacement therapy (–0.12; CI, –0.20 to –0.04; p < 0.01). Factors significantly associated with higher median antibiotics days included Medicare (0.92; CI, 0.29–1.55; p < 0.05) or private insurance (0.60; CI, 0.01–1.20; p = 0.05) compared with Medicaid or other government insurance, longer hospital LOS (0.42; CI, 0.38–0.47; p < 0.01), longer ICU days (0.17; CI, 0.06–0.28; p < 0.01), and more comorbidities (0.10; CI, 0.01–0.19; p = 0.026).
The odds of inhospital death (Table 4) did not differ after procalcitonin adoption when compared with before (odds ratio, 0.87; CI, 0.70–1.09; p = 0.234). Factors associated with increased odds of inhospital death included increased days of antibiotics use (1.04; CI, 1.01–1.07; p < 0.01), older age (1.01; CI, 1.01–1.02; p < 0.01), more comorbidities (1.23; CI, 1.17–1.29; p < 0.01), and longer days on mechanical ventilation (1.05; CI, 1.03–1.08; p < 0.01). Factors associated with decreased odds of inhospital death included Black race (0.73; CI, 0.56–0.96; p = 0.022) and longer hospital LOS (0.92; CI, 0.90–0.95; p < 0.01). The rate of readmission within 30 days did not differ in the post-procalcitonin adoption period when compared with pre-procalcitonin adoption (odds ratio, 0.77; CI, 0.59–1.01; p = 0.060). Higher number of comorbidities was the only factor associated with higher odds of 30-day readmissions (odds ratio, 1.06; CI, 1.00–1.13; p = 0.043).
TABLE 4.
The Association of Procalcitonin Test Availability for Physician Ordering in the Medical ICU of a Tertiary Health System on Inhospital Death, 30 Days Readmissions, and Indicated Charges (n = 1,793)
| Encounter Characteristics | Inhospital Death, OR (95% CI) | Thirty-d Same Facility Readmission (Excluding 502 Encounters With Inhospital Death), OR (95% CI) | Chargesa in $2020 |
|---|---|---|---|
| Procalcitonin adoption | |||
| Pre | Reference category | Reference category | Reference category |
| Post | 0.87 (0.70–1.10) | 0.77 (0.59–1.01) | –1,127.18b (–2,014.74 to –239.62) |
| Days of antibiotic used | 1.04c (1.01–1.07) | 1.01 (0.99–1.03) | 862.23c (544.15–1,180.32) |
| Gender | |||
| Male | Reference category | Reference category | Reference category |
| Female | 0.95 (0.76–1.19) | 0.95 (0.73–1.24) | –1,338.81c (–2,256.42 to –421.21) |
| Race | |||
| White | Reference category | Reference category | Reference category |
| Black | 0.73b (0.56–0.96) | 1.07 (0.78–1.46) | 802.73 (–214.58 to 1,820.08) |
| Asian or other | 1.00 (0.66–1.53) | 1.13 (0.69–1.86) | –65.93 (–1,506.81 to 1,374.94) |
| Ethnicity | |||
| Hispanic | Reference category | Reference category | Reference category |
| Non-Hispanic | 0.96 (0.63–1.45) | 0.76 (0.47–1.24) | –1,060.90 (–2,716.33 to 594.52) |
| Age | 1.02c (1.01–1.02) | 0.99 (0.98–1.00) | –25.29 (–59.82 to 9.24) |
| Insurance, Medicaid, or other government | Reference category | Reference category | Reference category |
| Medicare | 0.85 (0.59–1.24) | 1.22 (0.81–1.84) | –7.81 (–1,300.52 to 1,284.9) |
| Private | 1.39 (0.96–2.02) | 1.52 (0.99–2.31) | 190.15 (–1,083.92 to 1,464.22) |
| Self-pay or uninsured | 0.75 (0.26–2.16) | Not applicable | 2,103.45 (–2,340.77 to 6,547.66) |
| Hospital length of stay | 0.92b (0.90–0.95) | 1.00 (0.99–1.02) | –13.67 (–70.08 to 42.74) |
| ICU stay | 1.02 (0.99–1.05) | 0.98 (0.95–1.01) | 276.82c (134.75–418.89) |
| Number of Elixhauser comorbidities | 1.23c (1.17–1.29) | 1.06b (1.00–1.13) | 182.24 (–14.66 to 379.14) |
| Days on dialysis or renal replacement therapy | 1.01 (0.97–1.05) | 1.00 (0.97–1.03) | –401.51c (–552.83 to –250.18) |
| Days on mechanical ventilation | 1.05c (1.03–1.08) | 0.98 (0.96–1.00) | 35.87 (–49.83 to 121.59) |
| Discharge disposition | |||
| Home | Reference category | ||
| Facility | 980.55b (79.62–1,881.47) | ||
| Expired (inhospital) | 6,217.80c (4,124.89–8,310.70) | ||
OR = odds ratio.
aCharges include charges for procalcitonin, cultures, antibiotics, and vasoactive medications use.
bp < 0.05.
cp < 0.01.
Regression analysis adjusted for variables listed in table and clustered by patient level. Missing values for demographic characteristics were analyzed as a separate missing category.
Patients in the post-procalcitonin adoption period had a median 0.64 longer LOS compared with patients in the pre-procalcitonin period (coefficient, 0.64; CI, 0.12–1.15; p = 0.015). Factors significantly associated with longer median LOS included more days of antibiotics use (0.84; 0.78–0.90; p < 0.01), more ICU days (0.28; 0.16–0.39; p < 0.01), more comorbidities (0.27; CI, 0.15–0.39; p < 0.01), more days on renal replacement therapy (0.54; 0.26–0.83; p < 0.01), and more days on mechanical ventilation (0.22; CI, 0.14–0.29; p < 0.01). Factors associated with shorter median LOS (Supplemental Table, http://links.lww.com/CCX/A843) included Black race (–0.64; –1.20 to –0.08; p = 0.024) and inhospital death (–3.14; –3.72 to –2.57; p < 0.01).
Overall costs for procalcitonin, cultures, antibiotics, and vasoactive medications were less in the post-procalcitonin adoption period when compared with pre-procalcitonin. Patients post-procalcitonin adoption incurred $1,127.18 per patient less in total charges (–1,127.18; CI, –2,014.74 to –239.62; p = 0.013) after controlling for relevant factors. Factors significantly associated with higher charges in the post-procalcitonin period included more days of antibiotics use (862.23; CI, 544.15–1,180.32; p < 0.01), more ICU days (276.82; CI, 134.75–418.89; p < 0.01), discharged to facility (980.55; CI, 79.62–1,881.47; p = 0.033), or expired (6,217.80; CI, 4,124.89–8,310.70; p < 0.01) compared with discharged home. Factors significantly associated with lower charges for these services included female gender (–1,338.81; CI, –2,256.42 to –421.21; p < 0.01), and longer days on dialysis or renal replacement therapy (–401.51; –552.83 to –250.18; p < 0.01).
DISCUSSION
In our retrospective review of antibiotic management, costs, and outcomes after introduction of procalcitonin testing, we found that widespread adoption of procalcitonin testing was associated with reduced antibiotic use and overall costs without changing mortality or readmission rates.
Our findings add to a mixed literature regarding the clinical and economic effect of procalcitonin testing on sepsis management. While existing studies have generally observed that procalcitonin use reduces antibiotic use without negatively affecting patient outcomes (9–16), evidence regarding cost data is equivocal. Although many studies find clear financial savings with procalcitonin use (20–24), others find no reduction in costs (25–27). A large 2018 multicenter prospective randomized trial found no effect of procalcitonin testing on antibiotic use or adverse outcomes after implementing a procalcitonin-guided guideline in a large multicenter trial (28). However, patients in the trial were only enrolled after an initial primary diagnosis of LRTI was made. Our procalcitonin-based protocol differed in that all ICU admissions were included regardless of diagnosis (Supplemental Figure, http://links.lww.com/CCX/A842).
Several factors may explain the mixed evidence regarding the effect of procalcitonin testing on cost. For example, studies finding a lack of financial benefit may have included noncritically ill patients whose hospitalizations may not have been as costly and who may not have required aggressive use of antibiotics (26, 27). It is possible that procalcitonin testing has a greater impact in more critically ill patients. In addition, many studies limited the studied population to patients already admitted with a diagnosis of sepsis. We included all critically ill patients admitted to the ICU irrespective of associated diagnosis to improve generalizability and potentially affect the use of empiric antibiotics before a diagnosis of sepsis is made. Second, the financial relationship between the many stakeholders involved in the care of critically ill patients differs between countries as well as institutions. In this study, charge data was specific to the United States in one tertiary care hospital.
Our findings are hypothesis generating and may have implications for clinical care. Management of sepsis is often complex, and the optimal use and duration of antibiotic courses are unclear. Existing data often find that shorter courses may often result in equivalent outcomes to longer courses and suggest that antibiotic use can be safely reduced without affecting outcomes (29, 30). Such reductions have potential benefit in reducing morbidity from drug toxicity and in decreasing the occurrence of opportunistic infection and antibiotic-resistant organisms. Identifying the appropriate stopping point is even more important given quality protocols urging rapid administration of empiric antibiotics for presumed sepsis mortality (3–6). It was thus not surprising to find in our study that patients in the post-procalcitonin adoption period were less likely to receive antibiotics compared with patients during the pre-procalcitonin period. That we found no effect of procalcitonin use on mortality is also reasonable considering the lack of clear data regarding appropriate durations of antibiotic administration and the tendency of clinicians to treat until SIRS criteria normalize. Interestingly, there was a nonsignificant trend toward decreased odds of inhospital mortality in patients in the post-procalcitonin adoption period, with a statistically significant decreased odds in Black patients. In addition, we found longer lengths of stay in patients post-procalcitonin, suggesting that patients in that group may have had more severe illness. With the advent of new biomarkers to better diagnose sepsis, there is significant opportunity to improve sepsis management, antibiotic stewardship, and even improve cost-effective care. Further research is needed to best determine the appropriate use of procalcitonin testing in acute care.
Our study has several limitations. Because we used a before/after study design, it is possible that patients post-procalcitonin testing differed in terms of comorbidities and severity of illness to pre-procalcitonin or that hospital protocols have changed. There are limitations to procalcitonin testing itself, the diagnostic accuracy in patients who are immunocompromised has not been well studied as these patients have been generally excluded in randomized controlled trials. Additionally, procalcitonin levels may be higher in patients with advanced renal disease. This was a single-center study and cannot account for practice variation between institutions or internationally. In addition, the follow-up period was brief and did not account for a wash-out period given the short duration of this study. Also, as a pilot study for this specific population, a power analysis was not conducted. However, the opportunity to evaluate adoption of a new protocol at the time an inhospital assay for procalcitonin became available allowed for the introduction of a pilot study to generate new hypothesis. Future studies will need to account for these limitations to make the results more generalizable and plans for a larger study with longer-term follow-up are underway.
The results of this study show that in critically ill patients in a large U.S. tertiary care hospital, the adoption of a procalcitonin-based protocol for evaluation and treatment of sepsis may be associated with significant cost savings. As the most expensive condition treated in U.S. hospitals, these results may have important consequences for patients, hospitals, and policymakers.
CONCLUSIONS
In a single-center cost-effectiveness study, the adoption of a procalcitonin-based protocol to guide care was associated with an improvement in antibiotic stewardship and decreased costs in critically ill patients without adversely impacting clinical outcomes.
ACKNOWLEDGMENTS
This study was supported in part by grant from the Center for Health Outcomes and Informatics Research, Loyola University Chicago. We want to acknowledge the contribution of Ms. Susan Zelisko of Informatics and Clinical Research for conducting the data queries used in these analyses.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Chow and Bobay helped with the original idea, study design, article writing, editing drafts, and final submission. Dr. Markossian helped with the study design, article writing, editing drafts, and final submission. Dr. Albarillo helped with the original idea, article writing, editing drafts, and final submission. Dr. Donahey helped with the original idea, study design, and article writing.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Paoli CJ, Reynolds MA, Sinha M, et al. : Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018; 46:1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torio CM, Moore BJ: National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013. HCUP Statistical Brief #204. Rockville, MD, Agency for Healthcare Research and Quality, 2016. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf. Accessed October 2, 2019 [PubMed] [Google Scholar]
- 3.Kumar A, Roberts D, Wood KE, et al. : Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006; 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 4.Meehan TP, Fine MJ, Krumholz HM, et al. : Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997; 278:2080–2084 [PubMed] [Google Scholar]
- 5.Houck PM, Bratzler DW, Nsa W, et al. : Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004; 164:637–644 [DOI] [PubMed] [Google Scholar]
- 6.Gaieski DF, Mikkelsen ME, Band RA, et al. : Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010; 38:1045–1053 [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Sakhuja A, Kumar G, et al. : Culture-negative severe sepsis: Nationwide trends and outcomes. Chest. 2016; 150:1251–1259 [DOI] [PubMed] [Google Scholar]
- 8.Sigakis MJG, Jewell E, Maile MD, et al. : Culture-negative and culture-positive sepsis: A comparison of characteristics and outcomes. Anesth Analg. 2019; 129:1300–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assicot M, Gendrel D, Carsin H, et al. : High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993; 341:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbarth S, Holeckova K, Froidevaux C, et al. ; Geneva Sepsis Network: Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001; 164:396–402 [DOI] [PubMed] [Google Scholar]
- 11.Vijayan AL, Maya V, Ravindran S, et al. : Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017; 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markanday A: Acute phase reactants in infections: Evidence-based review and a guide for clinicians. Open Forum Infect Dis. 2015; 2:ofv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee C: Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017; 4:ofw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal R, Schwartz DN: Procalcitonin to guide duration of antimicrobial therapy in intensive care units: A systematic review. Clin Infect Dis. 2011; 53:379–387 [DOI] [PubMed] [Google Scholar]
- 15.Heyland DK, Johnson AP, Reynolds SC, et al. : Procalcitonin for reduced antibiotic exposure in the critical care setting: A systematic review and an economic evaluation. Crit Care Med. 2011; 39:1792–1799 [DOI] [PubMed] [Google Scholar]
- 16.Kutz A, Briel M, Christ-Crain M, et al. : Prognostic value of procalcitonin in respiratory tract infections across clinical settings. Crit Care. 2015; 19:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore BJ, White S, Washington R, et al. : Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ elixhauser comorbidity index. Med Care. 2017; 55:698–705 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services: Personal Health Care (PHC) Indices for All Services, Table 23 of the National Health Expenditures Accounts, Produced by the Centers for Medicare & Medicaid Services. Office of the Actuary, National Health Statistics Group. 2019. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. Accessed December 20, 2020
- 19.Dunn A, Grosse SD, Zuvekas SH: Adjusting health expenditures for inflation: A review of measures for health services research in the United States. Health Serv Res. 2018; 53:175–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon K, Suh JK, Jang EJ, et al. : Procalcitonin-guided treatment on duration of antibiotic therapy and cost in septic patients (PRODA): A multi-center randomized controlled trial. J Korean Med Sci. 2019; 34:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voermans AM, Mewes JC, Broyles MR, et al. : Cost-effectiveness analysis of a procalcitonin-guided decision algorithm for antibiotic stewardship using real-world U.S. hospital data. OMICS. 2019; 23:508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stojanovic I, Schneider JE, Wei L, et al. : Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: A Chinese hospital system perspective. Clin Chem Lab Med. 2017; 55:561–570 [DOI] [PubMed] [Google Scholar]
- 23.Balk RA, Kadri SS, Cao Z, et al. : Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017; 151:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison M, Collins CD: Is procalcitonin-guided antimicrobial use cost-effective in adult patients with suspected bacterial infection and sepsis? Infect Control Hosp Epidemiol. 2015; 36:265–272 [DOI] [PubMed] [Google Scholar]
- 25.Kip MMA, van Oers JA, Shajiei A, et al. : Cost-effectiveness of procalcitonin testing to guide antibiotic treatment duration in critically ill patients: Results from a randomised controlled multicentre trial in the Netherlands. Crit Care. 2018; 22:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KJ, Wateska A, Nowalk MP, et al. : Cost-effectiveness of procalcitonin-guided antibiotic use in community acquired pneumonia. J Gen Intern Med. 2013; 28:1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gluck E, Nguyen HB, Yalamanchili K, et al. : Real-world use of procalcitonin and other biomarkers among sepsis hospitalizations in the United States: A retrospective, observational study. PLoS One. 2018; 13:e0205924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang DT, Yealy DM, Filbin MR, et al. ; ProACT Investigators: Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018; 379:236–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawyer RG, Claridge JA, Nathens AB, et al. ; STOP-IT Trial Investigators: Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015; 372:1996–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugh R, Grant C, Cooke RP, et al. : Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2015; 2015:CD007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.