Abstract
Background
Previous research has documented that children conceived through medically assisted reproduction (MAR) are at increased risk of poor birth outcomes, such as low birthweight (LBW), which are risk factors for stunted longer-term cognitive development. However, parents who undergo MAR to conceive have, on average, advantaged socioeconomic backgrounds which could compensate for the negative effects of being born LBW. Previous studies have not analysed whether the negative effects of LBW are attenuated among MAR conceived children.
Methods
We draw on the UK Millennium Cohort Study (sweeps 1–6) which contains a sub-sample of (N = 396) MAR-conceived children. The dependent variable measures cognitive ability at around ages 3, 5, 7, 11 and 14. We examine the cognitive development of four groups of children: MAR-conceived low birthweight (MAR LBW); MAR-conceived non-low birthweight (MAR NLBW); naturally conceived low birthweight (NC LBW); naturally conceived non-low birthweight (NC NLBW). We estimate the two following linear regression models for each sweep: (i) a baseline model to examine the unadjusted association between cognitive development and low birthweight by mode of conception; and (ii) a model adjusted by socio-demographic family characteristics.
Results
In baseline models, MAR LBW children [age 3: β = 0.021, 95% confidence interval (CI): -0.198, 0.241; age 5: β = 0.21, 95% CI: 0.009, 0.418; age 7: β = 0.163, 95% CI: -0.148, 0.474; age 11: β = 0.003, 95% CI: -0.318, 0.325; age 14: β = 0.156, 95% CI: -0.205, 0.517], on average perform similarly in cognitive ability relative to NC NLBW at all ages, and display higher cognitive scores than NC LBW children until age 7. When we account for family characteristics, differences are largely attenuated and become close to zero at age 14.
Conclusions
Despite the higher incidence of LBW among MAR compared with NC children, they do not seem to experience any disadvantage in their cognitive development compared with naturally conceived children. This finding is likely explained by the fact that, on average, MAR children are born to socioeconomically advantaged parents.
Keywords: Medically assisted reproduction, low birthweight, LBW, cognitive development, UK cohort study
Key Messages
We investigate the cognitive development of children conceived through medically assisted reproduction (MAR) who are born low birthweight (LBW), compared with naturally conceived (NC) children who are born LBW and non-LBW (NLBW), from early infancy to mid-adolescence.
MAR LBW children, on average, perform similarly in cognitive development compared with NC NLBW children, and display higher cognitive scores compared with NC LBW children up to age 7.
When family characteristics are accounted for, we find that differences are largely attenuated and become close to zero at age 14.
MAR LBW children do not show any disadvantage in cognitive development compared with NC NLBW children, and family characteristics and resources may play a crucial role in hampering the effect of being born LBW.
Introduction
In Europe, the proportion of children born through medically assisted reproduction (MAR) nearly doubled during the first decade of the new millennium.1 Denmark has been a forerunner in this trend, with 9% of births being conceived through MAR in 2018.2 Other countries have also witnessed a remarkable increase in the number of children born after MAR. In the UK for example, the Human Fertilization and Embryology Authority reported that a total of 1238 MAR-conceived babies were born in 1991, 9423 in 2001 and 19 728 in 2018. The rapid increase in MAR conceptions and births over time has motivated research about its consequences for children’s well-being, in terms of both health at birth and later cognitive development.3,4
There is a large body of research showing that MAR children are at higher risk of experiencing adverse birth outcomes as compared with naturally conceived (NC) counterparts.5–7 MAR children are four times more likely to be born low birthweight (LBW) and three times more likely to be born premature.4 The mechanisms linking MAR to poor birth outcomes are not fully understood. Parental characteristics such as sub-fertility and advanced maternal age are factors that may lead parents to conceive using MAR, and they are considered risk factors for poor birth outcomes.6,8 At the same time, MAR techniques increase the chance of multiple births, which is also associated with poor birth outcomes 9. However, the difference in birth outcomes is only partially explained by the higher share of multiple birth among MAR-conceived children,9 as singleton births also experience higher risks of adverse birth outcomes.4 Overall, this evidence raises concerns regarding the longer-term cognitive development of MAR children, as poor birth outcomes such as low birthweight are linked to children’s future cognitive development10–14 through a number of biological mechanisms such as brain damage and hampered brain growth.15,16
Generally, studies show that MAR-conceived children perform better or similarly to naturally conceived children in terms of cognitive development.3,17–19 To date, however, no studies have examined how specifically MAR children born with low birthweight (LBW) fare relative to their peers. This is an important research gap, given the large share of MAR-conceived children experiencing poor birth outcomes, which are determinants of long-term health and socioeconomic well-being.12,13,20 We hypothesize that MAR children, regardless of whether they are born with poor birth outcomes, are advantaged relative to their naturally conceived peers. This may occur since parents who undergo MAR to conceive have, on average, a higher socioeconomic status (SES), which translates into higher cognitive scores among their children.21,22 In addition, there is evidence that poor birth outcomes are less salient in advantaged families as they have more resources to invest in their children, which may compensate for the initial health disadvantage.10,23
This study has two aims. First, we compare cognitive development across early infancy and mid-adolescence in four groups of children: MAR-conceived low birthweight (MAR LBW); MAR-conceived non-low birthweight (MAR NLBW); naturally conceived (NC) low birthweight (NC LBW); and naturally conceived non-low birthweight (NC NLBW). Although our focus is particularly to compare MAR LBW children with NC children (both LBW and NLBW), we also include MAR NLBW as we aim to provide a comprehensive perspective on children’s cognitive development based on their mode of conception and birthweight status. Second, we investigate whether family characteristics play a role in explaining these differences. We draw on the UK Millennium Cohort Study (sweeps 1–6), which includes a sub-sample of MAR-conceived and naturally conceived children and detailed information on both their cognitive development and family sociodemographic characteristics.
Methods
Sample description
We use data from the Millennium Cohort Study (MCS), which is a longitudinal cohort study following a sample of about 19 000 children born between 2000 and 2002 in England, Wales, Scotland and Northern Ireland. The first interview was conducted when the children were approximately 9 months old. Follow-up interviews were conducted in 2003, 2005, 2007, 2012 and 2015 when the cohort members were around 3, 5, 7, 11 and 14 years old. The sample of the MCS is randomly selected at the electoral ward level, and it is stratified to ensure representation of disadvantaged groups. Given the complex sample design, in the analyses we use weights to account for the over-representation of participants from ethnically diverse and disadvantaged areas. In the analyses, we use data from sweeps 1–6.
The analytical sample includes all the singletons and twins with valid information on the variables of interest at each sweep in which the outcome is measured (2–6). We drop the triplets (N = 30 in 10 families). These inclusion criteria leads us to exclude 6.58% (N = 966) of cases at age 3; 2% (N = 289) of cases at age 5; 3.46% (N = 455) at age 7; 2.39% (N = 302) at age 11; and 8.2% (N = 913) at age 14. The final analytical samples consist of: N = 13 716 observation at age 3; N = 14 175 at age 5; N = 12 714 observations at age 7; N = 12 336 at age 11; and N = 10 220 at age 14. We do not find any systematic association between our key independent variables (MAR-conceived and LBW) and the risk of not being included in the sample (see Supplementary Figure 1, available as Supplementary data at IJE online). We find limited evidence, however, that the analytical sample is positively selected since having a non-White or non-university educated mother slightly increases the chance of not being included at each sweep. Yet, we do not find any evidence that MAR children born from low-educated mothers are more likely to not be included. Thus, only NC children may be positively selected.
Variables
The outcome variable is children’s verbal cognitive ability assessed with the British Ability Scales (BAS II). The British Ability Scales II (BAS II) is a battery of twelve core sub-tests of cognitive ability and educational achievement24 and has demonstrated construct validity as a measure of cognitive ability24,25 and high test-retest reliability.25 Earlier work has found that BAS scores are associated with later educational attainment.26 One of the advantages of the BAS II is its flexibility, since the core sub-tests of the battery are individually interpretable. In order to assess a child’s level of performance, the child need not complete all of the tests in the battery.25 The BAS II is therefore particularly suitable for the collection of data in a survey setting where the time is limited; the MCS cohort members have only completed a sub-set of the BAS II sub-tests.27 Moreover, another main advantage is that children are presented with the test items that are most suitable for their age and ability, excluding items which are likely to be either too easy or too difficult. This works well in the MCS, where there is variation in children’s ages at the time of interview (sometimes even several months). The raw scores are then converted into ability scores which take into account the specific set of items the cohort members were presented with.27 At ages 3 and 5, children are assessed with the naming vocabulary scale; at age 7 with a word reading test; at age 11 with a verbal similarity test; and at age 14 with a word activity score. The scores are standardized (to a mean of 0 and a standard deviation of 1) and age-adjusted within each sweep (since there is variation in cohort members’ age within each sweep).
We have two key independent variables: (i) mode of conception (MAR or NC); and (ii) whether the child was born LBW. First, we define a child as conceived with MAR if the mother underwent one of the following treatments to conceive: in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), intrauterine insemination, or ovulation induction. The sample of MAR children consists of N = 396 children at age 3 and it reduces, because of attrition, to N = 296 at 14 years old. Despite the reduction of cases, the share of LBW children remains consistent across waves for both NC and MAR children (Supplementary Table 1, available as Supplementary data at IJE online). Second, following common practice in the literature, we define LBW as birthweight less than 2500 g.28 From this, we define four groups of children: MAR-conceived low birthweight (MAR LBW); MAR-conceived non-low birthweight (MAR NLBW); naturally conceived (NC) low birthweight (NC LBW); and NC non-low birthweight (NC NLBW).
We adjust the analyses for a series of confounders. As a measure of socioeconomic background, we use the level of education of the mother when the child was 6 months old. The variable is dichotomized between mothers with at least a university education (higher SES) and those with less than a university education (lower SES). We also conduct two sensitivity analyses: first, we replicated analyses using a detailed measure of maternal education; second, we used the highest occupational level among the parents (white collar vs others) measured with the National Statistics Socioeconomic Classification (NS-SEC), and results are consistent (see Supplementary Tables 2 and 3, available as Supplementary data at IJE online). As child characteristics, we adjust for sex, birth order (firstborn or higher) and twin births. As family characteristics, we adjust for maternal age at birth (continuous)8,29; marital status at birth (cohabiting/married and single); whether the mother accessed antenatal care before the 12th week of gestation; whether the mother is from an ethnic minority (White and non-White); and whether the mother smoked during pregnancy.30,31
Statistical models
We estimate two linear regression models at each sweep, which were collected at around ages 3, 5, 7, 11 and 14 years. First, we estimate baseline models predicting cognitive ability at each age for each of the four groups mentioned above, including only controls for sex and twin birth. Second, we estimate models adjusting for maternal education, birth order, maternal age, maternal marital status, timing of the first prenatal visit, maternal minority group and whether the mother smoked during pregnancy. All analyses were conducted using Stata Statistical Software: Release 16 (StataCorp LP, College Station, TX).
Results
Descriptive results
Table 1 presents the descriptive statistics of the analytical sample at age 3, for all births and by mode of conception. In the total analytical sample, 2.9% of the children are MAR-conceived. We find that—consistent with the existing evidence—the prevalence of LBW varies considerably by mode of conception; more than one-fifth (21.9%) of MAR-conceived children are LBW, whereas LBW is about 6% among NC children.
Table 1.
Child and family characteristics by mode of conception
| Total | NC | MAR | |
|---|---|---|---|
| Birth outcomes | |||
| LBW (%) | 7 | 6.6 | 21.9 |
| Child characteristics | |||
| Female (%) | 49.8 | 49.7 | 51.6 |
| Firstborn (%) | 42.3 | 41.5 | 66.1 |
| Twin (%) | 2.7 | 2.1 | 23.2 |
| Family characteristics | |||
| Maternal age at birth (mean/SE) | 29.7 (0.13) | 29.6 (0.12) | 33.1 (0.34) |
| Mother is married or cohabiting at birth (%) | 86.3 | 85.9 | 97.8 |
| Mother of White ethnic origin (%) | 90 | 89.9 | 94.5 |
| Mother has a university degree (%) | 33.5 | 33.1 | 45.5 |
| Mother smoked during pregnancy (%) | 21.4 | 21.8 | 9.3 |
| Mother used antenatal care before 12th week (%) | 43.3 | 42.9 | 55.4 |
| N (%) | 13716 (100) | 13320 (97.1) | 396 (2.9) |
Descriptive statistics refer to the analytical sample at age 3.
SE, standard error.
There are also differences in children’s characteristics by mode of conception. Among MAR-conceived children, 66% are firstborn as compared with 41% among NC children. Most notably, 23% of MAR-conceived children are born in a multiple birth, whereas the corresponding figure is only 2% of natural conceptions.
Family attributes also differ by mode of conception. Mothers who underwent MAR are on average almost 4 years older than mothers who conceived naturally. They are also more likely to be married or cohabit (98%) compared with NC mothers (85%). MAR mothers are also more likely to have a university degree compared with NC mothers, 45% and 33%, respectively. MAR mothers are also more likely to be White than NC mothers, a difference of about 5% age points. There are also differences in health behaviours, with MAR mothers being less likely to smoke during pregnancy and likely to access antenatal care earlier compared with mothers of NC children.
Regression analyses
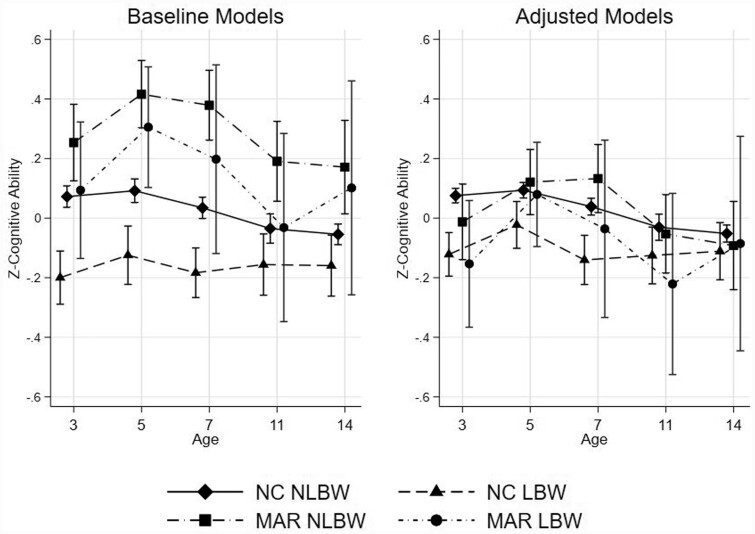
Table 2 shows the standardized regression coefficients [and 95% confidence intervals (CIs)] by mode of conception (MAR or NC) and weight status at birth (LBW or NLBW). The reference category is naturally conceived children born non-low birthweight (NC NLBW). The full model results are presented in Supplementary Tables 4 and 5, available as Supplementary data at IJE online. Figure 1 reports the predicted cognitive scores for the four groups.
Table 2.
Linear models regressing cognitive ability (standardized) on mode of conception and birthweight status
| Age 3 | Age 5 | Age 7 | Age 11 | Age 14 | |
|---|---|---|---|---|---|
| BAS | BAS | BAS | BAS | BAS | |
| naming vocabulary | naming vocabulary | word reading | verbal similarity | word activity | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Panel A: Baseline modelsa | |||||
| NC NLBW | Reference | Reference | Reference | Reference | Reference |
| NC LBW |
-0.272 (-0.348, -0.196) |
-0.216 (-0.306, -0.127) |
-0.218 (-0.303, -0.133) |
-0.121 (-0.210, -0.032) |
-0.105 (-0.208, -0.001) |
| MAR NLBW |
0.181 (0.052, 0.311) |
0.324 (0.211, 0.437) |
0.345 (0.232, 0.457) |
0.228 (0.093, 0.358) |
0.226 (0.068, 0.384) |
| MAR LBW |
0.021 (-0.198, 0.241) |
0.213 (0.009, 0.418) |
0.163 (-0.148, 0.474) |
0.003 (-0.318, 0.325) |
0.156 (-0.205, 0.517) |
| Panel B: Adjusted modelsb | |||||
| NC NLBW | Reference | Reference | Reference | Reference | Reference |
| NC LBW |
-0.200 (-0.267, -0.132) |
-0.118 (-0.196, -0.040) |
-0.182 (-0.266, -0.097) |
-0.093 (-0.179, -0.008) |
-0.062 (-0.161, 0.051) |
| MAR NLBW |
-0.067 (-0.192, -0.058) |
0.046 (-0.060, 0.153) |
0.115 (0.004, 0.226) |
0.002 (-0.130, 0.126) |
-0.028 (-0.180, 0.123) |
| MAR LBW |
-0.198 (-0.401, 0.005) |
0.017 (-0.159, 0.193) |
-0.074 (-0,369, 0.220) |
-0.162 (-0.472, 0.148) |
-0.023 (-0.386, 0.339) |
| N | 13716 | 14175 | 12714 | 12336 | 10220 |
NC NLBW, naturally conceived born non-low birthweight; NC LBW, naturally conceived born low birthweight; MAR NLBW, conceived with medically assisted reproduction and born non-low birthweight; MAR LBW: conceived with medically assisted reproduction and born low birthweight.
Baseline models include: sex, multiple birth.
Adjusted models include: sex, multiple birth, maternal education, whether the child is firstborn, maternal age at the time of birth, maternal marital status at the time of birth, timing of the first prenatal visit, ethnic origin, whether the mother smoked during pregnancy.
Figure 1.
Predicted scores in cognitive development and 95% confidence intervals of MAR and naturally conceived children by their weight status at birth at different age points. Baseline models include: sex, multiple birth. Adjusted models include: sex, multiple birth, maternal education, birth order, maternal age, maternal marital status, timing of the first prenatal visit, ethnic origin, whether the mother smoked during pregnancy. NC NLBW, naturally conceived born non-low birthweight; NC LBW, naturally conceived born low birthweight; MAR NLBW, conceived with medically assisted reproduction and born non-low birthweight; MAR LBW, conceived with medically assisted reproduction and born low birthweight.
Panel A of Figure 1 reports coefficients estimates from the baseline (or unadjusted) models, which include controls for the cohort members’ sex and multiple birth (Figure 1, left panel). MAR LBW children show equal or slightly higher cognitive scores compared with NC NLBW conceived children. At age 3, there are no differences between MAR LBW and NC NLBW children (β = 0.021, 95% CI: -0.198, 0.241). At age 5, MAR LBW children show higher cognitive ability scores (β = 0.21, 95% CI: 0.009, 0.418). Starting at age 7, the differences become smaller in magnitude and MAR LBW do not show notable differences in cognitive ability as compared with NC NLBW children (age 7: β = 0.163, 95% CI: -0.148, 0.474; age 11: β = 0.003, 95% CI: -0.318, 0.325; age 14: β = 0.156, 95% CI: -0.205, 0.517). When NC LBW children are used as the reference category (results not shown), the group showing the lowest cognitive ability scores at any age, MAR LBW display higher cognitive ability scores up to age 7 (at age 3: β = 0.293, 95% CI: 0.690, 0.517; at age 5: β = 0.430, CI: 0.218, 0.641; at age 7: β = 0.381, 95% CI: 0.069, 0.693); however, after age 7, the advantage is attenuated. MAR NLBW children show the highest predicted scores and higher scores than NC NLBW and NC LBW children at all ages, but compared with MAR LBW children, differences are smaller in magnitude, and the confidence intervals overlap at all ages.
Panel B reports coefficients for the adjusted models, in which we include controls for family sociodemographic characteristics (Figure 1, right panel). In contrast to the unadjusted estimates, we find that differences between NC and MAR (LBW and NLBW) conceived children become largely attenuated and close to zero at age 14. The only group of children showing a persistent negative cognitive performance are NC LBW children, who continue to show the lowest predicted cognitive ability until age 7.
Sensitivity analyses
We performed several sensitivity analyses. First, we replicated our analyses on the subgroup of MAR-children conceived with IVF and ICSI, since these procedures are more strongly associated with adverse birth outcomes.4,32 This analysis yielded similar results. Second, we restricted the analyses to only children included in all waves of the survey, to account for attrition in our analytical sample. Our results are unchanged. Third, research has recently questioned the use of LBW as an indicator of birth outcomes and developmental potential.33,34 We replicated analyses using an indicator of small for gestational age, and results are consistent. Fourth, we replicated our analyses using a more detailed definition of LBW, distinguishing very LBW (<1500 g) and LBW (between 1500 g and 2500 g), and our results are consistent. Finally, we re-performed the analyses controlling for maternal drinking in pregnancy, and results are consistent. The results of these sensitivity analyses are presented in Supplementary Tables 6–13, available as Supplementary data at IJE online.
Discussion
Medically assisted conceptions and births have increased considerably in recent decades, with over than 8 million MAR children born since the first treatment in the 1978.35 Previous research has shown that MAR-conceived children are at higher risk of being born with poor birth outcomes, such as low birthweight,4 raising concern about their future well-being including their cognitive development. Research has shown that MAR children perform as well as or better than naturally conceived children in cognitive ability, but one important aspect that has been overlooked is whether the MAR advantage is consistent for those born with LBW. MAR children, on average, come from socioeconomically advantaged backgrounds,36 which may compensate for the negative consequences of being born LBW.23 We used a representative UK longitudinal cohort study to investigate the cognitive development of MAR children who were born LBW compared with NC (low birthweight and non-low birthweight) children from infancy to mid-adolescence, before and after the adjustment for parental characteristics.
There are two main findings from this study. First, the results from the unadjusted models show that MAR-conceived children born LBW have similar or slightly higher cognitive ability scores compared with naturally conceived children. Specifically, MAR LBW children show cognitive ability scores that are similar to those of NC NLBW children and higher than NC LBW children up to age 7. Second, differences in cognitive ability between MAR and NC children are explained after adjustment for parental characteristics.
There are a range of mechanisms that potentially underlie our results. First, MAR parents’ characteristics, resources and behaviours may account for the MAR advantage.37 MAR parents could be more committed to parenting, due to the difficulty of conceiving thorough MAR and the desire to become parents.38 Moreover, since MAR parents are on average socioeconomically advantaged, they may give their children the chance to take part in a wider range of stimulating extra-curricular activities alongside school39;they may spend more time reading to their children40; they may provide their children with better child care (i.e. less day care and more adult-child interactions, which have long-lasting benefits in childhood)41—all of which are linked to positive cognitive development. Taken together, these characteristics and behaviours might enable MAR parents, by virtue of their higher SES, to compensate for the possible initial disadvantage of being born LBW.42,43 This is in line with research showing how poor birth outcomes are associated with cognitive impairments later in life primarily among families with a lower socioeconomic background.10,42
Second, it may be possible that we do not observe any disadvantage of being born LBW among MAR children, since the determinants of being born LBW differ between MAR and NC children. For example, in the MAR-conceived group, the risk of LBW may be more related to the experience of sub-fertility6 and less to other causes, such as unobserved health behaviours during pregnancy,44,45 which is more common among parents who conceive naturally. In line with this, the proportion of firstborn children—firstborn children are at higher risk of LBW than second- or later-born children46—in the MAR-conceived group is higher than in the NC group (66% vs 42%, see Table 1). The larger share of LBW children in the MAR group could be an indicator of physiological rather than pathological differences in birthweight. Ultimately, differences in the determinants of LBW could translate in differences in the developmental risk associated with being born LBW.
This study has also a secondary result. In the unadjusted models, we observe that MAR NLBW children have higher cognitive ability with respect to the NC group until age 7, when the gap is attenuated. Similarly, MAR LBW children show higher cognitive ability with respect to NC LBW children up to age 7, at which point the difference fully attenuates. These patterns may have two explanations. First, they could be explained by school entry, as schools may work as an equalizer for the cognitive development across groups.47 That is, the role of parents is more crucial at the earliest ages since they spend more time at home; once children enter schools, the influence of teachers and peers attenuates differences across groups. A similar pattern of narrowing differences in cognitive development is observed for SES gaps, where differences in cognitive ability between high- and low-SES children increase before entering school and remain stable thereafter.21,48 Second, it may be possible that we observe a convergence as the negative effect of being born LBW for NC conceived children fades away over time, which is consistent with previous studies documenting that the negative effect of LBW become smaller as children grow older.23,49,50
Our results partially differ from those of a previous study which has analysed the cognitive development of MAR children using the same data until age 11.3 Our results are consistent with theirs until age 7, after which they differ since we find that MAR NLBW show consistently higher cognitive ability with respect to all other groups before accounting for family characteristics. This difference is likely driven by the fact that we distinguish between NLBW and LBW children, whereas the other study considered MAR children in general.
This study is not free of limitations. First, the sample size of MAR children is small, which may affect the precision of our estimates. This is particularly the case in the later sweeps where, due to attrition, the confidence intervals become wider, making it difficult to interpret statistical differences between groups. Second, due to few children being conceived after MAR, we were required to combine all the children who were conceived through different MAR techniques, although the consequences of these procedures may differ.32 We repeated the analyses focusing only on a sub-sample of children conceived through IVF and ICSI (the most invasive techniques), and results were consistent. Third, our study focuses on the UK context, where the subsidization of MAR treatments through the National Health System is less generous compared with other contexts (such as Northern Europe), resulting in the majority of treatments taking place in the private sector and the MAR group being particularly selected. Results should be generalized to other contexts with caution, especially those where the subsidies are more generous.51 This study has nonetheless also considerable strengths. First, we investigate the consequences of MAR across children’s developmental stages, providing evidence on both early childhood and mid-adolescence, which are often understudied.17 Second, the richness of the data allows us to control for a large set of confounders, which enables us to further understand the mechanisms underlying the results.
In this study we show that, before adjustment for family characteristics which enable us to assess the actual well-being of different sub-population groups, MAR-conceived children born LBW do not show any disadvantage in cognitive development from infancy to mid-adolescence, but rather better cognitive performance than NC LBW and similar performance to NC NLBW children. The findings suggest that this is not a result of MAR per se, but because of selection into MAR and the advantaged profile of MAR families.
Our findings are important for two main reasons. First, the facts that the proportion of MAR-conceived children is increasing rapidly and a considerably high proportion are born LBW might constitute a concern and an impediment to their longer-term development. Overall, the findings provide a reassuring picture on the development of MAR-conceived children despite the increased risk of being born low birthweight. Second, by focusing on a subgroup of children born LBW to socially advantaged parents, our results corroborate earlier work showing that the impact of LBW on cognitive development is less consequential than the social context in which children are growing up.23
Future research should replicate the analyses using different data, especially in contexts—such as the Nordic countries—where the selection into MAR might be less pronounced because of a higher subsidization of MAR treatments through the public health system than in the UK. Future research should also further investigate if there are (and if so, which) family characteristics and behaviours specific to the MAR families which may be able to ameliorate the consequences of being born low birthweight.
Supplementary data
Supplementary data are available at IJE online.
Ethics approval
Ethical approval was not required as the study used anonymized, publicly available data.
Funding
This work was supported by European Research Council agreement no. 803959 (to A.G.), by Economic and Social Research Council grant ES/M001660/1, and by funding from the Academy of Finland (decision number 324613).
Supplementary Material
Acknowledgements
We gratefully acknowledge the constructive comments and suggestions from the editors and anonymous reviewers. We thank 2001 MCS families for their time and cooperation, as well as the Centre for Longitudinal Studies at the Institute of Education at University College London. We are grateful to the Centre for Longitudinal Studies (CLS), UCL Social Research Institute, for the use of these data and to the UK Data Service for making them available. However, neither CLS nor the UK Data Service bear any responsibility for the analysis or interpretation of these data.
Author contributions
A.G. with M.C. and S.A. conceptualized the study. M.C. performed the statistical analyses, S.A. and A.G. advised on the study design and interpretation of the results. M.C. wrote the first draft. S.A. and A.G. read, reviewed and revised the manuscript. All the authors contributed to subsequent drafts. All the authors approved the final manuscript.
Data availability
Data and code are available upon reasonable request to the corresponding author.
Conflict of interest
We have no conflict of interest to declare.
References
- 1. Ferraretti AP, Nygren K, Andersen AN. et al. Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod Open 2017;2017:hox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martins MV, Vassard D, Hougaard CØ, Schmidt L.. The impact of ART on union dissolution: a register-based study in Denmark 1994–2010. Hum Reprod 2018;33:434–40. [DOI] [PubMed] [Google Scholar]
- 3. Barbuscia A, Mills MC.. Cognitive development in children up to age 11 years born after ART—a longitudinal cohort study. Hum Reprod 2017;32:1482–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goisis A, Remes H, Martikainen P, Klemetti R, Myrskylä M.. Medically assisted reproduction and birth outcomes: a within-family analysis using Finnish population registers. Lancet 2019;393:1225–32. [DOI] [PubMed] [Google Scholar]
- 5. Martin AS, Chang J, Zhang Y. et al. Perinatal outcomes among singletons after assisted reproductive technology with single-embryo or double-embryo transfer versus no assisted reproductive technology. Fertil Steril 2017;107:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinborg A, Wennerholm U-B, Romundstad L. et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- 7. Sutcliffe AG, Ludwig M.. Outcome of assisted reproduction. Lancet 2007;370:351–59. [DOI] [PubMed] [Google Scholar]
- 8. Goisis A, Remes H, Barclay K, Martikainen P, Myrskylä M.. Advanced maternal age and the risk of low birthweight and preterm delivery: a within-family analysis using Finnish population registers. Am J Epidemiol 2017;186:1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ESHRE Task Force on Ethics and Law. 6. Ethical issues related to multiple pregnancies in medically assisted procreation. Hum Reprod 2003;18:1976–79. [DOI] [PubMed] [Google Scholar]
- 10. Torche F. Prenatal exposure to an acute stressor and children's cognitive outcomes. Demography 2018;55:1611–39. [DOI] [PubMed] [Google Scholar]
- 11. Heckman JJ. The economics, technology, and neuroscience of human capability formation. Proc Natl Acad Sci U S A 2007;104:13250–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Black SE, Devereux PJ, Salvanes KG.. From the cradle to the labor market? The effect of birthweight on adult outcomes. Q J Econ 2007;122:409–39. [Google Scholar]
- 13. Härkönen J, Kaymakçalan H, Mäki P, Taanila A.. Prenatal health, educational attainment, and intergenerational inequality: the Northern Finland Birth Cohort 1966 Study. Demography 2012;49:525–52. [DOI] [PubMed] [Google Scholar]
- 14. Almond D, Currie J, Duque V.. Childhood circumstances and adult outcomes: Act II. J Econ Lit 2018;56:1360–446. [Google Scholar]
- 15. Abernethy LJ, Palaniappan M, Cooke RW.. Quantitative magnetic resonance imaging of the brain in survivors of very low birthweight. Arch Dis Child 2002;87:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hack M, Klein NK, Taylor HG.. Long-term developmental outcomes of low birthweight infants. Future Child 1995;5:176–96. [PubMed] [Google Scholar]
- 17. Bay B, Mortensen EL, Kesmodel US.. Assisted reproduction and child neurodevelopmental outcomes: a systematic review. Fertil Steril 2013;100:844–53. [DOI] [PubMed] [Google Scholar]
- 18. Shankaran S. Outcomes from infancy to adulthood after assisted reproductive technology. Fertil Steril 2014;101:1217–21. [DOI] [PubMed] [Google Scholar]
- 19. Carson C, Kelly Y, Kurinczuk JJ, Sacker A, Redshaw M, Quigley MA.. Effect of pregnancy planning and fertility treatment on cognitive outcomes in children at ages 3 and 5: longitudinal cohort study. BMJ 2011;343:d4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Figlio D, Guryan J, Karbownik K, Roth J.. The effects of poor neonatal health on children's cognitive development. Am Econ Rev 2014;104:3921–55. [DOI] [PubMed] [Google Scholar]
- 21. Skopek J, Passaretta G.. The evolution of social and ethnic inequalities in cognitive achievement from preschool to secondary schooling in the UK. In: Passaretta G, Skopek J, (eds). Roots and Development of Achievement Gaps. A Longitudinal Assessment in Selected European Countries, ISOTIS Report (D 1.3), Trinity College Dublin, 2018. [Google Scholar]
- 22. Gil-Hernández CJ. Do well-off families compensate for low cognitive ability? evidence on social inequality in early schooling from a twin study. Sociol Educ 2019;92:150–75. [Google Scholar]
- 23. Boardman JD, Powers DA, Padilla YC, Hummer RA.. Low birthweight, social factors, and developmental outcomes among children in the United States. Demography 2002;39:353–68. [DOI] [PubMed] [Google Scholar]
- 24. Elliot C, Smith P, McCulloch K, British Ability Scales – Second Edition: Administration and Scoring Manual. Windsor, UK: NFER, 1996. [Google Scholar]
- 25. Elliot C, Smith P, McCulloch K, British Abilities Scale Second Edition (BAS II) Technical Manual. Windsor, UK: NFER-Nelson, 1997. [Google Scholar]
- 26. Feinstein L. Inequality in the early cognitive development of British children in the 1970 cohort. Economica 2003;70:73–97. [Google Scholar]
- 27. Connelly R. Interpreting test scores. Institute of Education, Discussion Paper. London: UCL Institute of Education, 2013.
- 28. Torche F, Conley DA.. Pound of flesh: the use of birthweight as a measure of human capital endowment in economics research. In: Komlos J, Kelly I, eds. The Oxford Handbook of Economics and Human Biology. New York, NY: Oxford University Press, 2016. [Google Scholar]
- 29. Goisis A, Schneider DC, Myrskylä M.. Secular changes in the association between advanced maternal age and the risk of low birthweight: a cross-cohort comparison in the UK. Popul Stud (Camb) 2018;72:1–17. [DOI] [PubMed] [Google Scholar]
- 30. Lien DS, Evans WN.. Estimating the impact of large cigarette tax hikes the case of maternal smoking and infant birthweight. J Hum Resour 2005;40:373–92. [Google Scholar]
- 31. Grandjean P, Landrigan PJ.. Neurobehavioural effects of developmental toxicity. Lancet Neurol 2014;13:330–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knoester M, Helmerhorst FM, Vandenbroucke JP. et al. Cognitive development of singletons born after intracytoplasmic sperm injection compared with in vitro fertilization and natural conception. Fertil Steril 2008;90:289–96. [DOI] [PubMed] [Google Scholar]
- 33. Conti G, Hanson MA, Inskip H, Crozier S, Cooper C, Godfrey K, Beyond Birthweight: The Origins of Human Capital. IFS Working Papers. LondonInstitute for Fiscal Studies, 2018.
- 34. Grätz M, Torche F.. Compensation or reinforcement? the stratification of parental responses to children’s early ability. Demography 2016;53:1883–904. [DOI] [PubMed] [Google Scholar]
- 35. Calhaz-Jorge C, de Geyter C, Kupka MS. et al. ; European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2012: results generated from European registers by ESHRE. Hum Reprod 2016;31:1638–52. [DOI] [PubMed] [Google Scholar]
- 36. Goisis A, Sigle-Rushton W.. Childbearing postponement and child well-being: a complex and varied relationship? Demography 2014;51:1821–41. [DOI] [PubMed] [Google Scholar]
- 37. Bratsberg B, Rogeberg O, Skirbekk V.. Fathers of children conceived using ART have higher cognitive ability scores than fathers of naturally conceived children. Hum Reprod 2020;35:1461–68. [DOI] [PubMed] [Google Scholar]
- 38. Golombok S, Brewaeys A, Cook R. et al. Children: the European study of assisted reproduction families: family functioning and child development. Hum Reprod 1996;11:2324–31. [DOI] [PubMed] [Google Scholar]
- 39. Larreau A Unequal Childhoods: Class, Race, and Family Life: Berkeley and LosAngeles, CA: University of California Press, 2011. [Google Scholar]
- 40. Barone C, Fougère D, Martel K, Reading aloud to children, social inequalities, and vocabulary development: evidence from a randomized controlled trial. SSRN. https://ssrn.com/abstract=3648798, 14 Jul 2020, preprint: not peer reviewed
- 41. Fort M, Ichino A, Zanella G.. Cognitive and noncognitive costs of day care at age 0–2 for children in advantaged families. J Polit Econ 2020;128:158–205. [Google Scholar]
- 42. Almond D, Edlund L, Palme M.. Chernobyl's subclinical legacy: prenatal exposure to radioactive fallout and school outcomes in Sweden. Q J Econ 2009;124:1729–72. [Google Scholar]
- 43. Bernardi F. Compensatory advantage as a mechanism of educational inequality: A regression discontinuity based on month of birth. Sociol Educ 2014;87:74–88. [Google Scholar]
- 44. Härkönen J, Lindberg M, Karlsson L, Karlsson H, Scheinin NM.. Education is the strongest socioeconomic predictor of smoking in pregnancy. Addiction 2018;113:1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kramer MS, Séguin L, Lydon J, Goulet L.. Socio‐economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol 2000;14:194–210. [DOI] [PubMed] [Google Scholar]
- 46. Hinkle SN, Albert PS, Mendola P. et al. The association between parity and birthweight in a longitudinal consecutive pregnancy cohort. Paediatr Perinat Epidemiol 2014;28:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Downey DB, Von Hippel PT, Broh BA.. Are schools the great equalizer? Cognitive inequality during the summer months and the school year. Am Sociol Rev 2004;69:613–35. [Google Scholar]
- 48. Skopek J, Passaretta G. Socioeconomic Inequality in Children’s Achievement from Infancy to Adolescence: The Case of Germany. Soc Forces2020:1–27. [Google Scholar]
- 49. Grossman M. On the concept of health capital and the demand for health. J Polit Econ 1972;80:223–55. [Google Scholar]
- 50. Goisis A, Özcan B, Myrskylä M.. Decline in the negative association between low birthweight and cognitive ability. Proc Natl Acad Sci U S A 2017;114:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chambers GM, Adamson GD, Eijkemans MJ.. Acceptable cost for the patient and society. Fertil Steril 2013;100:319–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available upon reasonable request to the corresponding author.