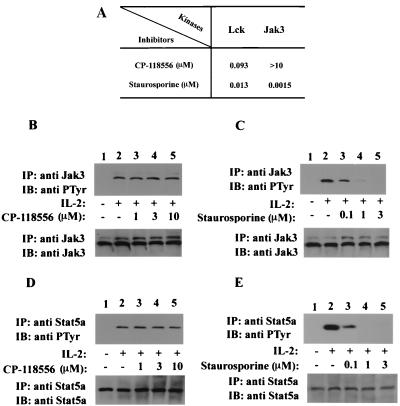
FIG. 2.
Inhibition of Lck does not block IL-2-induced phosphorylation of Jak3 and Stat5a. (A) In vitro catalytic activity of Lck and Jak3 was detected by ELISA using random copolymers of l-glutamic acid and tyrosine as the substrate. Prior to the kinase assay, each well was incubated with an appropriate concentration of ATP and dilutions of the indicated kinase inhibitors, after which recombinant kinase domains of Lck and Jak3 were added. Values represent 50% inhibitory concentrations of CP-118556 and staurosporine for Lck and Jak3. (B to E) YT cells were incubated without (lanes 1 and 2) or with (lanes 3 to 5) inhibitors at 37°C (30 min), followed by IL-2 stimulation (15 min, lanes 2 to 5). The cells were lysed and immunoprecipitated with anti-Jak3 (B and C) or anti-Stat5a (D and E) antiserum. The immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antiphosphotyrosine (anti PTyr) MAb 4G10 (B to E, upper panels), anti-Jak3 antiserum (B and C, lower panels), and anti-Stat5a antiserum (D and E, lower panels).