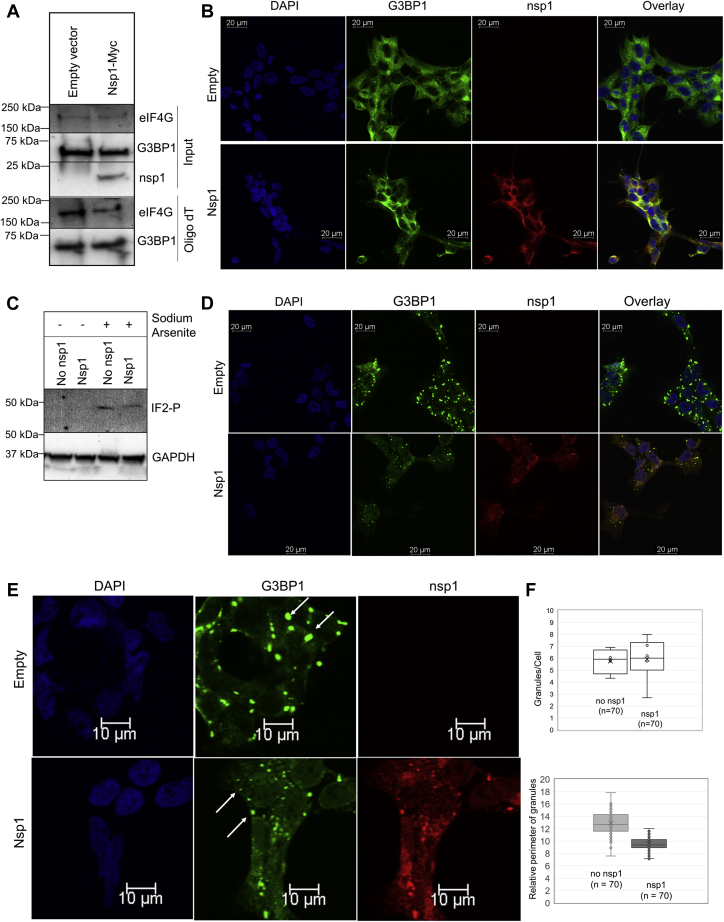
Figure 4.
Sodium arsenite–induced stress granule formation in the presence and absence of nsp1 identifies colocalization of G3BP1 and nsp1.A, association of eIF4G and G3BP1 to mRNA was analyzed in the presence of nsp1 from cells expressing nsp1-Myc. OligodT pull down was conducted from cells with and without nsp1, followed by immunoblot analysis of G3BP1 and eIF4G pulled down by OligodT. B, HEK cells were transfected with pCAGGS-nsp1-Myc. Cells were fixed after 24 h, and protein localization was observed using anti-G3BP1 and anti-nsp1 antibodies. Images were captured using Leica TCS SP8 confocal microscope with a 40× objective. C, cells undergoing sodium arsenite treatment were analyzed for phosphorylation of eIF2α and GAPDH (control) using immunoblot. D, stress was induced by 0.5 mM NaAsO2 for 40 min, and cells were fixed and probed with anti-G3BP1 and anti-Myc antibodies. E, a zoomed-in representation of stress granules in cells with and without nsp1-Myc. F, the quantification of stress granules was done using ImageJ software. The relative perimeter was calculated in micromolar (3.52 pixel/μm). eIF, eukaryotic translation initiation factor; G3BP1, Ras GTPase-activating protein SH3 domain–binding protein 1; HEK, human embryonic kidney cell line; nsp1, nonstructural protein 1.