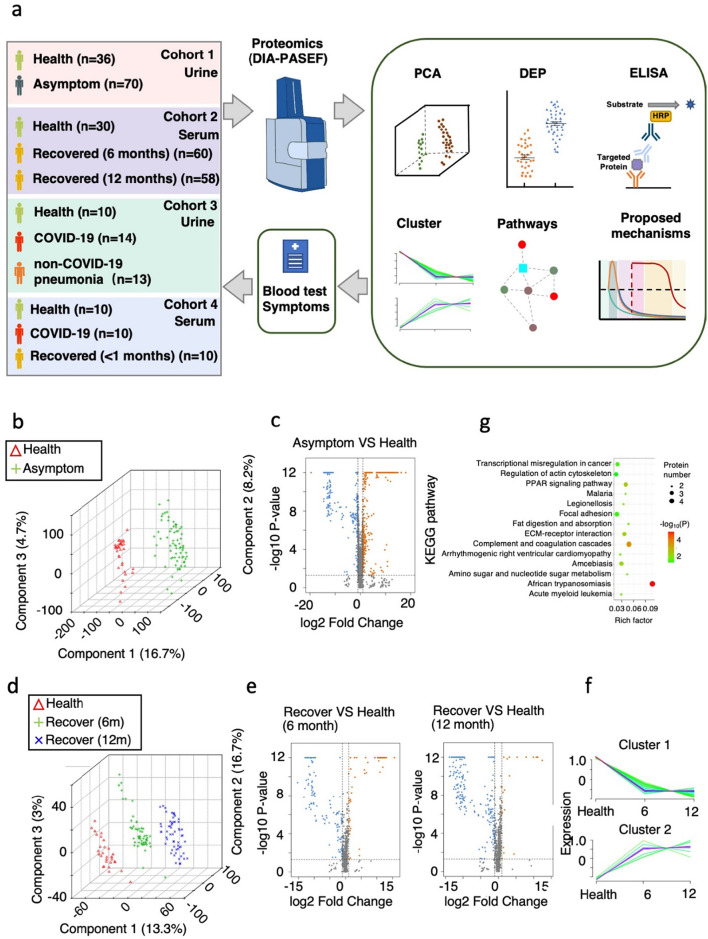
Fig. 1.
Summary of COVID-19 patient samples and experimental design.
a. Experimental design for the quantitative proteomic analysis in this study. In cohort 1, a total of 106 urine samples were analyzed from 2 groups: healthy controls, n = 36; asymptomatic carriers, n = 70. In cohort 2, a total of 148 serum samples were taken from 3 groups: healthy controls, n = 30; convalescent patients (7 severe cases) recovered for 6 months, n = 60; convalescent patients (7 severe cases) recovered for 12 months, n = 58. The cohort 3 and 4 are data from other studies. All differentially expressed proteins (DEPs) selected for further analysis meet the criteria that fold change >2 or < 0.5, two-tailed t-test; p < 0.05).
b. Principal components analysis (PCA) showing the inter-group differences in cohort 1. Individuals in the healthy control group and the asymptomatic carrier group are indicated by coloured symbols in the figure.
c. Volcano plot of identified urine DEPs comparing asymptomatic carriers with the healthy control group in cohort 1 (two-tailed t-test; p < 0.05, fold change >2 or < 0.5).
d. Principal components analysis (PCA) showing the inter-group differences in cohort 2. Individuals in the healthy control group, and patients recovered for 6 or 12 months are indicated by coloured symbols in the figure.
e. Volcano plot of identified serum DEPs comparing patients recovered for 6 or 12 months with the healthy control group (cohort 2).
f. Hierarchical clustering shows 2 distinct groups differentiated according to similarity.
g. KEGG-based enrichment analysis of DEPs in the subclusters shown in f. KEGG terms were sorted by P value.