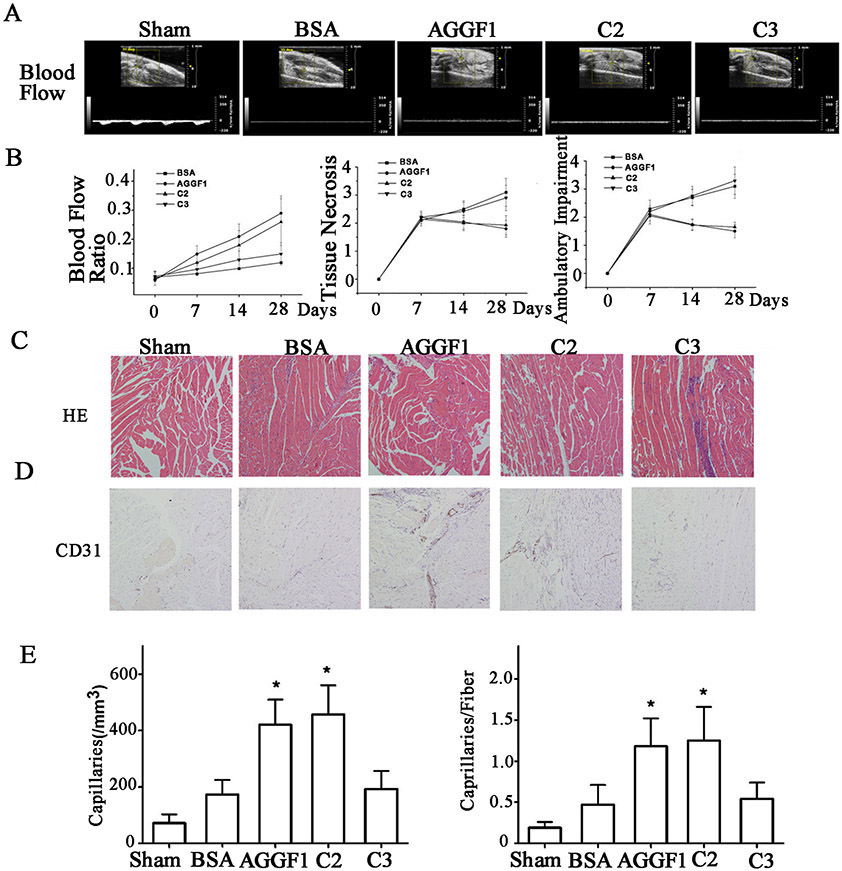
Figure 8. Analysis of WT AGGF1 and mutant C2 with the functional angiogenic domain, and mutant C3 without the domain in male mice.
(A) The treatment effect of WT and mutant AGGF1 proteins for PAD in a hindlimb ischemia mouse model. Representative Doppler ultrasound images are shown for male mice treated with WT AGGF1, mutant C2, and mutant C3. Sham mice and BSA were used as negative controls. The structure of mutants C2 and C3 can be found in Figure 4A.
(B) The ratios of blood flow in the ischemic leg over that in the non-ischemic limb, tissue necrosis scores and ambulatory impairment scores are shown for different time points in days. The blood flow was measured by high resolution micro-ultrasound (left panel). Effects of different treatments on tissue necrosis are shown in the middle panel. Effects of different treatments on ambulatory impairment are shown in the right panel. Histological examinations of muscle tissue.
(C) Representative H&E staining images of sections of ischemic hindlimb muscles.
(D) Representative immunostaining images of sections of ischemic hindlimb muscles stained with an anti-CD31 antibody.
(E) Quantification of density of CD-31-positive vessels per mm2 in ischemic muscles 28 days after different treatments (left panel). The number of CD-31-positive vessels per muscular fiber is plotted for different treatments for the time point of 28 days (right panel).
All data are shown as mean ± SEM. *P≤0.05, n=13/group (one-way ANOVA with Dunnett post hoc tests).